
(a)
Interpretation:
The structural formula for the given compound is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.7E
The structural formula for the given compound is shown below.
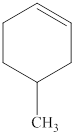
Explanation of Solution
The given compound is

Figure 1
The structural formula of the given compound is shown in figure 1.
(b)
Interpretation:
The structural formula for the given compound is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.7E
The structural formula for the given compound is shown below.
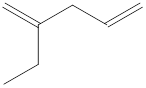
Explanation of Solution
The given compound is

Figure 2
The structural formula of the given compound is shown in figure 2.
(c)
Interpretation:
The structural formula for the given compound is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.7E
The structural formula for the given compound is shown below.
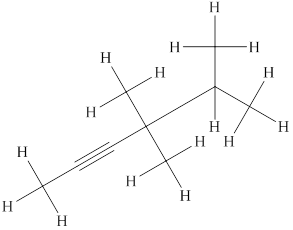
Explanation of Solution
The given compound is

Figure 3
The structural formula of the given compound is shown in figure 3.
(d)
Interpretation:
The structural formula for the given compound is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.7E
The structural formula for the given compound is shown below.
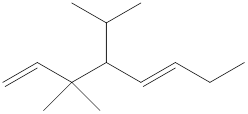
Explanation of Solution
The given compound is

Figure 4
The structural formula of the given compound is shown in figure 4.
(e)
Interpretation:
The structural formula for the given compound is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.7E
The structural formula for the given compound is shown below.

Explanation of Solution
The given compound is

Figure 5
The structural formula of the given compound is shown in figure 5.
(f)
Interpretation:
The structural formula for the given compound is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.7E
The structural formula for the given compound is shown below.
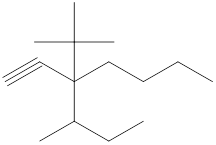
Explanation of Solution
The given compound is

Figure 6
The structural formula of the given compound is shown in figure 6.
Want to see more full solutions like this?
Chapter 12 Solutions
Study Guide with Student Solutions Manual for Seager/Slabaugh/Hansen's Chemistry for Today: General, Organic, and Biochemistry, 9th Edition
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





