
Concept explainers
Interpretation:
The structural formulas and the IUPAC names for the
Concept introduction:
The systematic naming of organic compound is given by
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.9E
The structural formulas and the IUPAC names of the
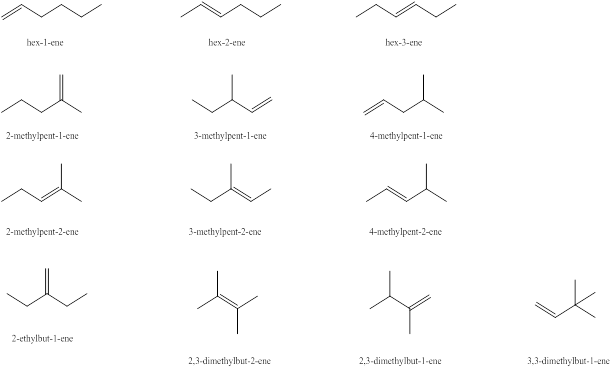
Explanation of Solution
The first alkene isomer of
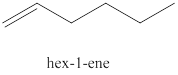
Figure 1
The second alkene isomer of
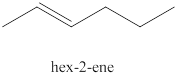
Figure 2
The third alkene isomer of
Figure 3
The fourth alkene isomer of
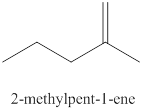
Figure 4
The fifth alkene isomer of
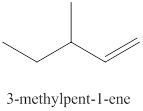
Figure 5
The sixth alkene isomer of
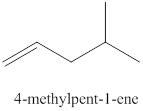
Figure 6
The seventh alkene isomer of
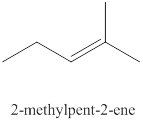
Figure 7
The eighth alkene isomer of
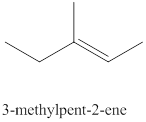
Figure 8
The ninth alkene isomer of
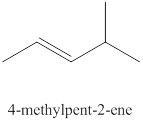
Figure 9
The tenth alkene isomer of
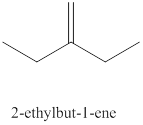
Figure 10
The eleventh alkene isomer of
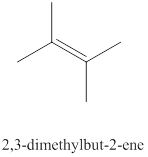
Figure 11
The twelfth alkene isomer of
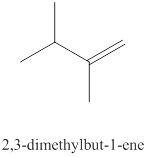
Figure 12
The thirteenth alkene isomer of
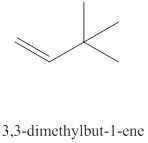
Figure 13
The structural formulas and the IUPAC names of the
Want to see more full solutions like this?
Chapter 12 Solutions
Study Guide with Student Solutions Manual for Seager/Slabaugh/Hansen's Chemistry for Today: General, Organic, and Biochemistry, 9th Edition
- :0: :0: Select to Add Arrows :0: (CH3)2NH :0: ■ Select to Add Arrows :0: :0: (CH3)2NH ■ Select to Add Arrowsarrow_forwardDraw the product of the following H action sequence. Ignore any inorganic byproducts formed. 1. (CH3CH2)2CuLi, THF 2. CH3Br Q Atoms, Bonds and Rings H Charges ㅁarrow_forwardPlease help me with this the problem is so confusingarrow_forward
- 14 Question (1 point) Disiamylborane adds to a triple bond to give an alkenylborane. Upon oxidation with OH, H2O2, the alkenylborane will form an enol that tautomerizes to an aldehyde. In the first box below, draw the mechanism arrows for the reaction of disiamylborane with the alkyne, and in the last box draw the structure of the aldehyde. 4th attempt Feedback i > 3rd attempt OH, H2O2 i See Periodic Table See Hintarrow_forwardanswer with mechanisms and steps. handwritten please!arrow_forwardHello I need some help with Smartwork. For drawing structure B, I know the correct answer is CH₃B₂, but when I try to type it in, it keeps giving me CH₄BH₃ instead. Do you know how I should write it properly? Should I use a bond or something else?arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





