
(a)
Interpretation:
The structural formula for the given compound is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.6E
The structural formula for the given compound is shown below.
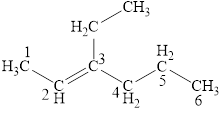
Explanation of Solution
The given compound is

Figure 1
The structural formula of the given compound is shown in figure 1.
(b)
Interpretation:
The structural formula for the given compound is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.6E
The structural formula for the given compound is shown below.
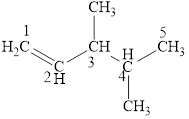
Explanation of Solution
The given compound is

Figure 2
The structural formula of the given compound is shown in figure 2.
(c)
Interpretation:
The structural formula for the given compound is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.6E
The structural formula for the given compound is shown below.
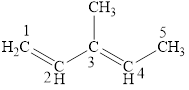
Explanation of Solution
The given compound is

Figure 3
The structural formula of the given compound is shown in figure 3.
(d)
Interpretation:
The structural formula for the given compound is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.6E
The structural formula for the given compound is shown below.
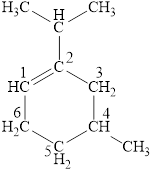
Explanation of Solution
The given compound is

Figure 4
The structural formula of the given compound is shown in figure 4.
(e)
Interpretation:
The structural formula for the given compound is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are:
• First identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if same type of substituents are present.
• Name the substituents in alphabetical order.
Answer to Problem 12.6E
The structural formula for the given compound is shown below.
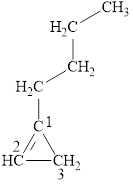
Explanation of Solution
The given compound is

Figure 5
The structural formula of the given compound is shown in figure 5.
Want to see more full solutions like this?
Chapter 12 Solutions
Bundle: Chemistry For Today: General, Organic, And Biochemistry, 9th + Owlv2 With Mindtap Reader, 1 Term (6 Months) Printed Access Card
- Provide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forward
- If I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forwardFeedback (7/10) Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. Incorrect, 3 attempts remaining Ο (CH3CH2)2NH, TSOH Select to Draw V N. 87% Retryarrow_forward
- If I want to obtain (1,1-dipropoxyethyl)benzene from 1-bromopropene, indicate the product that I have to add in addition to NaOH.arrow_forwardIndicate the products obtained when fluorobenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained when chlorobenzene acid reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
- Indicate the products obtained by reacting benzenesulfonic acid with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained by reacting ethylbenzene with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained when tert-butylbenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning



