
(a)
Interpretation:
How the given compound can be produced from an alkene is to be shown.
Concept introduction:
Electrophilic addition of carbenes to
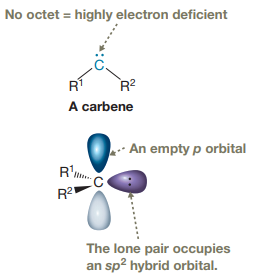
Carbenes are typically highly electrophilic due to the lack of an octet and are
Answer to Problem 12.2P
The given compound can be produced from an alkene is shown below:
Explanation of Solution
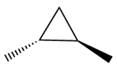
The given compound is:
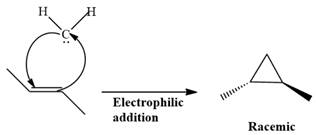
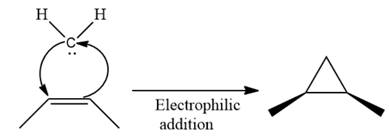
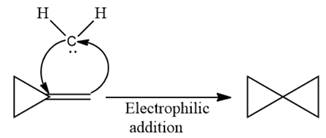
Electrophilic addition of carbenes to alkenes forms cyclopropane rings. Diazomethane (
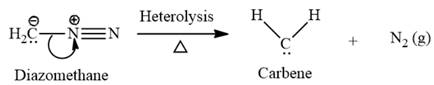
This carbene intermediate reacts with an alkene in a concerted one-step reaction to form a cyclopropyl ring. The bridge carbon atom in the cyclopropyl ring comes from the carbene while the two carbon atoms are from the original alkene. Two new
![]()
The complete reaction of the addition of carbene to alkene is shown below:
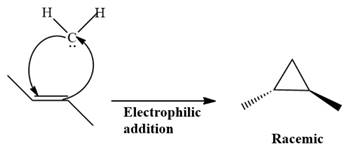
The given compound is produced via an electrophilic addition reaction of a carbene to an alkene.
(b)
Interpretation:
How the given compound can be produced from an alkene is to be shown.
Concept introduction:
Electrophilic addition of carbenes to alkenes forms cyclopropane rings. In carbine, the carbon atom has two bonds and a lone pair of electrons.

Carbenes are typically highly electrophilic due to the lack of an octet and are
Answer to Problem 12.2P
The given compound can be produced from an alkene is shown below:

Explanation of Solution
The given compound is:
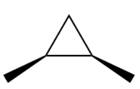
Electrophilic addition of carbenes to alkenes forms cyclopropane rings. Diazomethane (
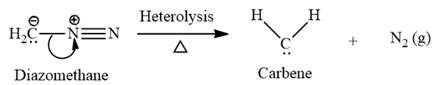
This carbene intermediate reacts with an alkene in a concerted one-step reaction to form a cyclopropyl ring. The bridge carbon atom in the cyclopropyl ring comes from the carbene while the two carbon atoms are from the original alkene. Two new
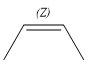
The complete reaction of the addition of carbene to alkene is shown below:
The given compound is produced via an electrophilic addition reaction of a carbene to an alkene.
(c)
Interpretation:
How the given compound can be produced from an alkene is to be shown.
Concept introduction:
Electrophilic addition of carbenes to alkenes form cyclopropane rings. In carbine, the carbon atom has two bonds and a lone pair of electrons.

Carbenes are typically highly electrophilic due to the lack of an octet and are
Answer to Problem 12.2P
The given compound can be produced from an alkene is shown below:

Explanation of Solution
The given compound is:
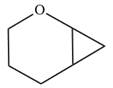
Electrophilic addition of carbenes to alkenes forms cyclopropane rings. Diazomethane (
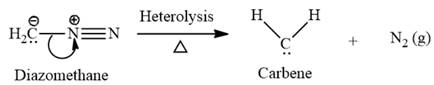
This carbene intermediate reacts with an alkene in a concerted one-step reaction to form a cyclopropyl ring. The bridge carbon atom in the cyclopropyl ring comes from the carbene while the two carbon atoms are from the original alkene. Two new
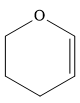
The complete reaction of the addition of carbene to alkene is shown below:
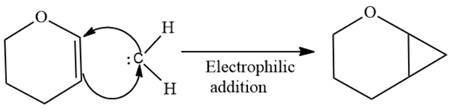
The given compound is produced via an electrophilic addition reaction of a carbene to an alkene.
(d)
Interpretation:
How the given compound can be produced from an alkene is to be shown.
Concept introduction:
Electrophilic addition of carbenes to alkenes forms cyclopropane rings. In carbine, the carbon atom has two bonds and a lone pair of electrons.

Carbenes are typically highly electrophilic due to the lack of an octet and are
Answer to Problem 12.2P
The given compound can be produced from an alkene is shown below:
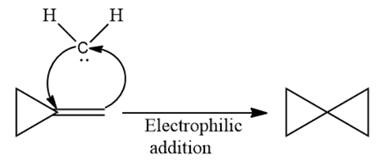
Explanation of Solution
The given compound is:

Electrophilic addition of carbenes to alkenes forms cyclopropane rings. Diazomethane (
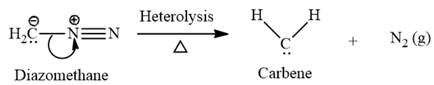
This carbene intermediate reacts with an alkene in a concerted one-step reaction to form a cyclopropyl ring. The bridge carbon atom in the cyclopropyl ring comes from the carbene while the two carbon atoms are from the original alkene. Two new
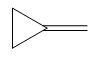
The complete reaction of the addition of carbene to alkene is shown below:
The given compound is produced via an electrophilic addition reaction of a carbene to an alkene.
Want to see more full solutions like this?
Chapter 12 Solutions
Get Ready for Organic Chemistry
- HELP NOW PLEASE ! ASAP! URGENT!arrow_forwardHELP NOW PLEASE ! ASAP! URGENT!arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward
- Pls help.arrow_forward13) When solid barium phosphate is in equilibrium with its ions, the ratio of barium ions to phosphate ions would be: a. 1:1 b. 2:3 c. 3:2 d. 2:1 14) The pH of a 0.05 M solution of HCl(aq) at 25°C is 15) The pH of a 0.20 M solution of KOH at 25°C isarrow_forwardPls help.arrow_forward
- Pls help.arrow_forward16) A 2.0 L flask containing 2.0 x 10-3 mol H2(g), 3.0 x 10-3 mol Cl2(g), and 4.0 x 10-3 mol HCl(g) at equilibrium. This system is represented by the following chemical equation: H2 (g) + Cl2 (g) → 2HCl(g) Calculate the equilibrium constant for this reaction.arrow_forward7) The pH of a 0.05M solution of HCl(aq) at 25°C is a. 1.3 b. 2.3 c. 3.3 d. 12.7arrow_forward
- 11) The Ksp expression for copper (II) sulfate is: a. [Cu2+][SO4²¯] b. [Cu²+]² [SO4²]² c. [Cu²+]²[SO4²] d. [CuSO4] 12) Which of the following is true about a chemical system in equilibrium? a. All chemical reactions have stopped b. The concentration of reactants is equal to the concertation of products c. The forward and reverse reaction rates become equal d. The system will remain at equilibrium regardless of any external factorsarrow_forward21) Explain the difference between the rate of a reaction and the extent of a reaction. Why are both of these concepts important, if you are a chemical engineer that is trying to develop a process to produce a large volume of a specific type of chemical compound?arrow_forwardPls help.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

