
Organic Chemistry Plus Mastering Chemistry with Pearson eText -- Access Card Package (9th Edition) (New in Organic Chemistry)
9th Edition
ISBN: 9780321971128
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.23SP
An unknown, foul-smelling hydrocarbon gives the mass spectrum and infrared spectrum shown.
- a. Use the mass spectrum to propose a molecular formula. How many elements of unsaturation are there?
- b. Use the IR spectrum to determine the
functional group (s), if any. - c. Propose one or more structures for this compound. What parts of the structure are uncertain? If you knew that hydrogenation of the compound gives n-octane, would the structure still be uncertain?
- d. Propose structures for the major fragments at 39, 67, 81, and 95 in the mass spectrum.
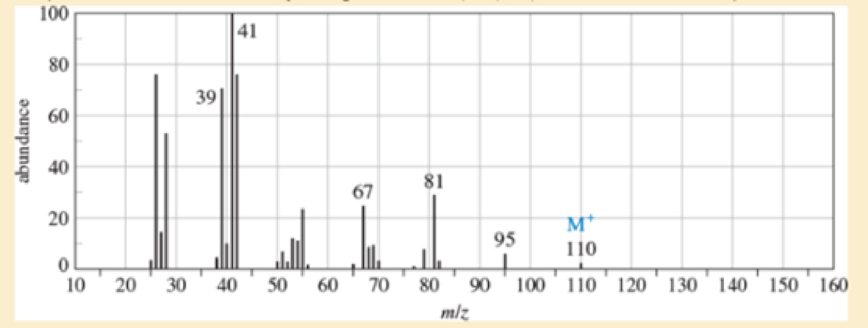
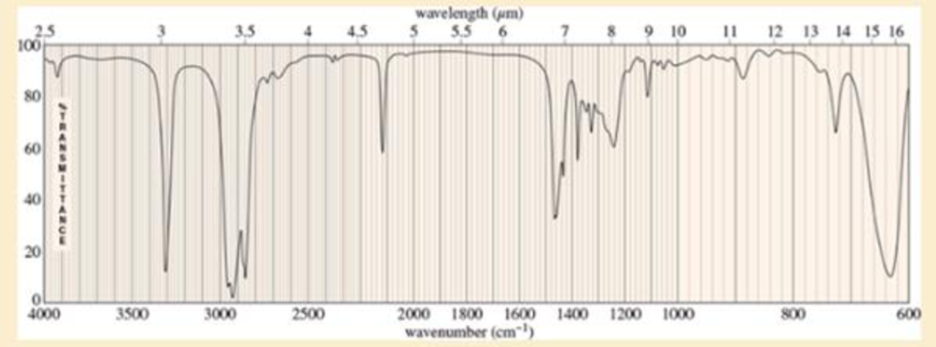
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please label this HNMR
Consider the following gas chromatographs of Compound A, Compound B, and a mixture of Compounds A and B.
Inject
A
B
mixture
Area= 9
Area = 5
Area = 3
Area
Inject
.
མི།
Inject
J2
What is the percentage of Compound B in the the mixture?
Rank these according to stability.
CH3
H3C
CH3
1
CH3
H3C
1 most stable, 3 least stable
O 1 most stable, 2 least stable
2 most stable, 1 least stable
O2 most stable, 3 least stable
O3 most stable, 2 least stable
O3 most stable, 1 least stable
CH3
2
CH3
CH3
H₂C
CH3
3
CH3
CH
Chapter 12 Solutions
Organic Chemistry Plus Mastering Chemistry with Pearson eText -- Access Card Package (9th Edition) (New in Organic Chemistry)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Additional Science Textbook Solutions
Find more solutions based on key concepts
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider this IR and NMR: INFRARED SPECTRUM TRANSMITTANCE 0.8- 0.6 0.4 0.2 3000 10 9 8 00 HSP-00-541 7 CO 6 2000 Wavenumber (cm-1) сл 5 ppm 4 M Which compound gave rise to these spectra? N 1000 1 0arrow_forwardConsider this reaction (molecular weights are under each compound): HC=CH + 2 HCI --> C2H4Cl 2 MW = 26 36.5 99 If 4.4 g of HC=CH are reacted with 110 mL of a 2.3 M HCI solution, and 6.0 g of product are actually produced, what is the percent yield?arrow_forwardWhat is the name of the major product of this reaction? OH CH3 H₂SO4, heat 1-methylcyclohexene O2-methyl-1-cyclohexene O 3-mthylcyclohexene 1-methyl-2-cyclohexenearrow_forward
- We added a brown solution of Br2 to one of our products, and the brown color disappeared. This indicated that our product wasarrow_forwardRank the following according to reactivity toward nitration: a) benzene b) bromobenzene c) nitrobenzene d) phenol Od) greatest, c) least Od) greatest, b) least Od) greatest, a) least a) greatest, b) least a) greatest, c) least Oa) greatest, d) least Ob) greatest, a) least O b) greatest, c) least Ob) greatest, d) least O c) greatest, a) least O c) greatest, b) least O c) greatest, d) leastarrow_forwardO-Nitrophenol was distilled over with the steam in our experiment while the other isomer did not. This is due to: O intramolecular hydrogen bonding in the ortho isomer O intermolecular hydrogen bonding in the the ortho isomer O the ortho isomer has a lower density O the ortho isomer has a lower molecular weightarrow_forward
- K 44% Problem 68 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :6: :: :CI: CI CI: :0:0 Select to Add Arrows Select to Add Arrows H H Cl CI: CI CI: Select to Add Arrows Select to Add Arrows H :CI: Alarrow_forwardI I H :0: Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. 0:0 :0: CI ΑΙ :CI: :CI: :0: CI Select to Add Arrows Select to Add Arrows cl. :0: Cl © ハ CI:: CI H CO Select to Add Arrows Select to Add Arrows 10: AI ::arrow_forwardOrder the following compounds from slowest to fastest in a nucleophilic acyl substitution reaction. ii 요 OB D A E C OCE Darrow_forwardI need the most help figuring out how to find [I^-] mol/ L, [S2O8^2-] mol/L. 1st and 2nd Blank columns.arrow_forwardCan someone help me whats the issue?arrow_forwarda. The change in the Gibbs energy of a certain constant pressure process is found to fit the expression: AG-85.1 J mol −1 +36.5 J mol ¹K-1 × T A. Calculate the value of AS for the process. B. Next, use the Gibbs-Helmholtz equation: (a(AG/T)) ΔΗ - T2 to calculate the value of AH for the process.arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY