
(a)
Interpretation:
The Lewis structure for given acetone and carbon disulfide molecules should be determined and the deviation from ideal behavior should be explained using intermolecular forces.
Concept Introduction:
- The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
- Sometimes the
chemical bonding of a molecule cannot be represented using a single Lewis structure. In these cases, the chemical bonding are described by delocalization of electrons and is known as resonance.
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
Intermolecular force: The attractive force that withholds two molecules is called as intermolecular force. The influence of intermolecular forces depends on molar mass and the
Types and strength of intermolecular forces in decreasing order:
Polar molecule: Polar molecules have large dipole moments.
Non-Polar molecules: Non-polar molecules have bonded atoms with similar electronegativity results to have zero dipole moments.
(a)
Explanation of Solution
The Lewis structures are as follows,
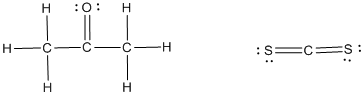
The given molecule acetone is polar whereas the molecule carbon disulfide is nonpolar molecule.
According to Like dissolves like principle, the attraction between acetone and
Due to weak attractions the molecules will leave the solution that result in increased vapor pressure of the solution than the sum of vapor pressures by Raoutl’s law.
(b)
Interpretation:
The vapor pressure of the solution under ideal condition should be determined.
Concept Introduction:
Raoult’s law: Raoult’s law states that solvent partial pressure over a solution is equal to vapor pressure of pure solvent multiplied with mole fraction of the solvent.
Where,
The solution that obeys Raoult’s law is defined as ideal solution.
(b)
Explanation of Solution
The ideal solution should obey Raoult’s law.
Partial pressure for A is as follows,
Partial pressure for B is as follows,
The ideal pressure of the solution is
(c)
Interpretation:
The sign of
Concept Introduction:
Enthalpy
Enthalpy is the amount energy absorbed or released in a process.
The enthalpy change in a system
Where,
(c)
Explanation of Solution
The given molecule acetone is polar whereas the molecule carbon disulfide is nonpolar molecule.
According to Like dissolves like principle, the attraction between acetone and
Due to weak attractions the molecules will leave the solution that result in increased vapor pressure of the solution than the sum of vapor pressures by Raoutl’s law.
Therefore, the solution deviates positively from Raoutl’s law which says that heat of solution is positive that means mixing is an endothermic process (heat is absorbed by the system).
Want to see more full solutions like this?
Chapter 12 Solutions
Chemistry
- Draw the products of the stronger acid protonating the other reactant. དའི་སྐད”“ H3C OH H3C CH CH3 KEq Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C NH2 NH2 KEq H3C-CH₂ 1. Product acid Product basearrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forward
- Draw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forwardDraw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forward
- Draw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). H-Br CH2Cl2arrow_forwardWrite the aldol condensation mechanism and product for benzaldehyde + cyclohexanone in a base. Then trans-cinnamaldehyde + acetone in base. Then, trans-cinnamaldehyde + cyclohexanone in a base.arrow_forwardClick the "draw structure" button to launch the drawing utility. Draw the structure of the alkene that yields the following set of oxidative cleavage products? draw structure ...arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning  Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





