
(a)
Interpretation:
Whether the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The
Therefore,
The
(b)
Interpretation:
Whether the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The
Therefore,
The
(c)
Interpretation:
Whether the cyclohexane ring “breathing” or simultaneous stretching of the all
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The simultaneous stretch of all
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The simultaneous stretch of all
Therefore, simultaneous stretch of all
The simultaneous stretch of all
(d)
Interpretation:
Whether the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The
Therefore,
The
(e)
Interpretation:
Whether the symmetrical stretching of the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The symmetric stretch of
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The symmetric stretch of
Therefore, symmetric stretch of
The symmetric stretch of
(f)
Interpretation:
Whether the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The stretch of
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The stretch of
Therefore, stretch of
The stretch of
(g)
Interpretation:
Whether the
Concept introduction:
The IR rays are able to stretch the bond in the compound as they carry the energy sufficient to perform this task. The compound is said to be IR active if the energy absorbed the bond leads to change in the dipole moment of the molecule.
Answer to Problem 12.10P
The
Explanation of Solution
Change in the dipole moment of the molecule on IR absorption is the necessary condition to be IR active.
The structure of
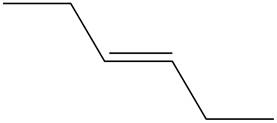
Figure 1
The
Therefore,
The
Want to see more full solutions like this?
Chapter 12 Solutions
EBK ORGANIC CHEMISTRY STUDY GUIDE AND S
- Soap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forward
- Provide the mechanism for this transformation: *see imagearrow_forwardAssign all the signals individually (please assign the red, green and blue)arrow_forwardThe two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forward
- .. Give the major organic product(s) for each of the following reactions or sequences of reactions. Show ll relevant stereochemistry [3 ONLY]. A H Br 1. NaCN 2 NaOH, H₂O, heat 3. H3O+ B. CH₂COOH 19000 1. LiAlH4 THF, heat 2 H₂O* C. CH Br 1. NaCN, acetone 2 H3O+, heat D. Br 1. Mg. ether 3. H₂O+ 2 CO₂ E. CN 1. (CH) CHMgBr, ether 2 H₂O+arrow_forwardAssign this COSY spectrumarrow_forwardCan I please get help with this?arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
