
Concept explainers
a)

Interpretation:
A structure for the molecule which meets the descriptions given is to be assigned.
C6H12O; IR: 1715 cm-1; 13CNMR: 8.0 δ (10), 18.5 δ (10), 33.5 δ (20), 40.6 δ (30), 214.0 δ (40)
Concept introduction:
In 13CNMR, carbons in double bond which are sp2 hybridized absorb from 110 to 220 δ. Saturated aldehydes and ketones usually absorb in the region from 200 to 215 δ While aromatic and unsaturated carbonyl compounds absorb in the 190 to 200 δ region. The primary alkyl carbon absorbs in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ. The signals will be observed at downfield if these alkyl groups are attached to electron withdrawing groups.
To assign:
A structure for the molecule which meets the descriptions given.
C6H12O; IR: 1715 cm-1; 13CNMR: 8.0 δ (10), 18.5 δ (10), 33.5 δ (20), 40.6 δ (30), 214.0 δ (40)
Answer:
Answer to Problem 69AP
A structure for the molecule which meets the spectral descriptions given is
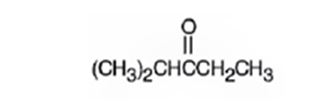
Explanation of Solution
The molecular formula of the compound is C6H12O. Ignoring oxygen the molecular formula of the parent compound becomes C6H14. Thus the compound has two hydrogen atoms (14-12) less than the parent and hence has one unsaturation unit perhaps a C=O.
The IR absorption at 1715 cm-1 and 13CNMR peak at 214.0 δ indicate that the compound is a ketone. The 13CNMR further indicates that the carbonyl group is flanked by a secondary and tertiary carbon indicated by the two downfield signals at 33.5 δ and 40.6 δ. The remaining three carbons has to be primary, of which one is attached as methyl(8.0 δ) to the secondary carbon and two others are attached as two methyl groups (18.5δ) on the tertiary carbon. Hence the compound is 2-methyl-3-pentanone.
A structure for the molecule which meets the spectral descriptions given is

b)

Interpretation:
A structure for the molecule which meets the descriptions given is to be assigned.
C5H10O; IR: 1730 cm-1; 13CNMR: 22.6 δ (10), 23.6 δ (30), 52.8 δ (20), 202.4 δ (30).
Concept introduction:
Aldehydes and ketones show a strong absorption band in IR from 1660-1770 cm-1. Aldehydes show two characteristic C-H absorptions between 2700-2760cm-1 and 2800-2860 cm-1. Saturated aldehydes absorb near 1730 cm-1 while aromatic aldehydes and α, β- unsaturated aldehydes absorb near 1705 cm-1. Saturated ketones and cyclohexanones absorb near 1715 cm-1 while aromatic ketones and α, β- unsaturated ketones absorb near 1685-1690 cm-1. Cyclopentanones absorb around 1750 cm-1.
In 13CNMR, carbons in double bond which are sp2 hybridized absorb from 110 to 220 δ. Saturated aldehydes and ketones usually absorb in the region from 200 to 215 δ While aromatic and unsaturated carbonyl compounds absorb in the 190 to 200 δ region. The primary alkyl carbon absorbs in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ. The signals will be observed at downfield if these alkyl groups are attached to electron withdrawing groups.
To assign:
A structure for the molecule which meets the descriptions given.
C5H10O; IR: 1730 cm-1; 13CNMR: 22.6 δ (10), 23.6 δ (30), 52.8 δ (20), 202.4 δ (30).
Answer to Problem 69AP
A structure for the molecule which meets the spectral descriptions given is

Explanation of Solution
The molecular formula of the compound is C6H12O. Ignoring oxygen the molecular formula of the parent compound becomes C6H14. Thus the compound has two hydrogen atoms less than the parent and hence has one unsaturation unit, a carbonyl group.
The IR absorption at 1730 cm-1 and 13CNMR peak at 202.4 δ indicate that the compound is a saturated aldehyde. The 13CNMR further indicates that the carbonyl group is attached to a secondary carbon indicated by the downfield signal at 52.8 δ. Of the remaining three carbons one has to be tertiary(23.6 δ) which is attached to two methyl groups(22.6 δ). Hence the compound is 2-methylbutanal.
A structure for the molecule which meets the spectral descriptions given is

c)
Interpretation:
A structure for the molecule which meets the descriptions given is to be assigned.
C6H8O; IR: 1680 cm-1; 13CNMR: 22.9 δ (20), 25.8 δ (20), 38.2 δ (20), 129.8 δ (30) 150.6 δ (30), 198.7 δ (40).
Concept introduction:
Aldehydes and ketones show a strong absorption band in IR from 1660-1770 cm-1. Aldehydes show two characteristic C-H absorptions between 2700-2760cm-1 and 2800-2860 cm-1. Saturated aldehydes absorb near 1730 cm-1 while aromatic aldehydes and α, β- unsaturated aldehydes absorb near 1705 cm-1. Saturated ketones and cyclohexanones absorb near 1715 cm-1 while aromatic ketones and α, β- unsaturated ketones absorb near 1685-1690 cm-1. Cyclopentanones absorb around 1750 cm-1.
In 13CNMR, carbons in double bond which are sp2 hybridized absorb from 110 to 220 δ. Saturated aldehydes and ketones usually absorb in the region from 200 to 215 δ While aromatic and unsaturated carbonyl compounds absorb in the 190 to 200 δ region. The primary alkyl carbon absorbs in the range 10-15 δ, a secondary alkyl radical in the range 16-25 δ while a tertiary alkyl in the range 25-35 δ.
To assign:
A structure for the molecule which meets the descriptions given.
C6H8O; IR: 1680 cm-1; 13CNMR: 22.9 δ (20), 25.8 δ (20), 38.2 δ (20), 129.8 δ (30) 150.6 δ (30), 198.7 δ (40).
Answer to Problem 69AP
A structure for the molecule which meets the spectral descriptions given is
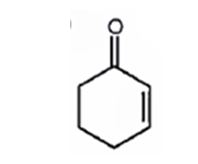
Explanation of Solution
The molecular formula of the compound is C6H8O. Ignoring oxygen the molecular formula of the parent compound becomes C6H14. Thus the compound has six hydrogen atoms (14-8= 6) less than the parent and hence has three unsaturation units.
The IR absorption at 1680 cm-1 and 13CNMR peak at 198.7 δ indicate that the compound is an α, β- unsaturatedketone. The compound has three unsaturated units. α, β- unsaturatedketone accounts for two unsaturation units. Hence the compound contains a six membered ring. The signals at 129.8 δ (30) 150.6 δ (30) can be attributed to the carbons in double bond. The other three signals at 22.9 δ (20), 25.8 δ (20), 38.2 δ (20) can be attributed to three methylene groups. The signal at 38.2 δ (20) can be assigned to methylene carbon attached to carbonyl carbon, that at 25.8 δ (20) to the methylene carbon carbon attached to C4 while that at 22.9 δ (20) is due to methylene carbon C5.
A structure for the molecule which meets the spectral descriptions given is

Want to see more full solutions like this?
Chapter 11 Solutions
EBK ORGANIC CHEMISTRY
- What alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forward
- Draw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward
- 3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). H-Br CH2Cl2arrow_forwardWrite the aldol condensation mechanism and product for benzaldehyde + cyclohexanone in a base. Then trans-cinnamaldehyde + acetone in base. Then, trans-cinnamaldehyde + cyclohexanone in a base.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

