
a)
Interpretation:
The given molecule has to be prepared by using nucleophilic substitution reaction.
Concept introduction:
SN1 reaction:
The alcohol is reaction with acids like hydrochloric acid or hydrobromic acid which yield the corresponding carbocation intermediate, this carbocation intermediate undergoes substitution reaction which yields the corresponding substitution product.
Tertiary alcohols undergo substitution very fast than the secondary alcohols because tertiary carbocation is more stable than the secondary carbocation than the primary carbocation.
Primary alcohol is less stable therefore it won’t undergo SN1substitution reaction.
SN2 reaction:
The alcohol is reaction with acids like hydrochloric acid or hydrobromic acid, the bromine atom attacks back side of the carbon atoms in simultaneous manner and which is bearing alcohol group which yield the corresponding product.
Example:
Alcohol is reaction with tosyl chloride in pyridine which provides retention of configuration of tosylated compound. This tosylated compound is further reaction with sodium methoxide which undergoes again SN2 type of reaction, the methoxide ion attacks the carbon atom through the back side and provides Inverse configuration of methoxy compound. This is shown below,
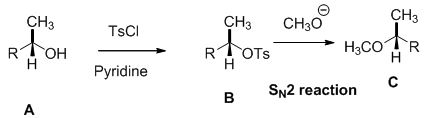
SN2 reaction is second order
Answer to Problem 45AP
The reaction is given below,
Explanation of Solution
Given information:
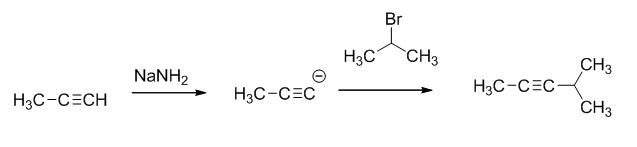
The product of the reaction is given below,
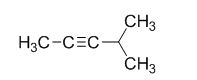
The reaction is given below,
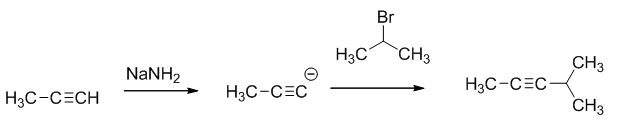
The sodium amide is acts as a base and it abstract the highly acidic proton from propylene gives carbanion, this carbanion react with 2-bromo propane gives the corresponding product.
The given molecule is prepared by using nucleophilic substitution reaction.
b)
Interpretation:
The given molecule has to be prepared by using nucleophilic substitution reaction.
Concept introduction:
SN1 reaction:
The alcohol is reaction with acids like hydrochloric acid or hydrobromic acid which yield the corresponding carbocation intermediate, this carbocation intermediate undergoes substitution reaction which yields the corresponding substitution product.
Tertiary alcohols undergo substitution very fast than the secondary alcohols because tertiary carbocation is more stable than the secondary carbocation than the primary carbocation.
Primary alcohol is less stable therefore it won’t undergo SN1substitution reaction.
SN2 reaction:
The alcohol is reaction with acids like hydrochloric acid or hydrobromic acid, the bromine atom attacks back side of the carbon atoms in simultaneous manner and which is bearing alcohol group which yield the corresponding product.
Example:
Alcohol is reaction with tosyl chloride in pyridine which provides retention of configuration of tosylated compound. This tosylated compound is further reaction with sodium methoxide which undergoes again SN2 type of reaction, the methoxide ion attacks the carbon atom through the back side and provides Inverse configuration of methoxy compound. This is shown below,

SN2 reaction is second order reaction, the rate of the reaction is depending on the both substrate and nucleophiles.
Answer to Problem 45AP
The reaction is given below,
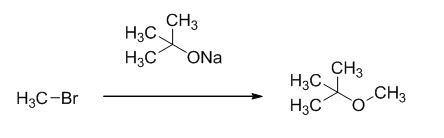
Explanation of Solution
Given information:
The product of the reaction is given below,
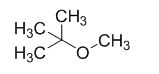
The reaction is given below,
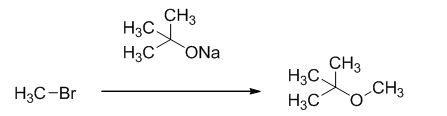
The sodium tertiary butoxide is acts as a base and it react with methyl bromide gives the corresponding ether product.
The given molecule is prepared by using nucleophilic substitution reaction.
c)
Interpretation:
The given molecule has to be prepared by using nucleophilic substitution reaction.
Concept introduction:
SN1 reaction:
The alcohol is reaction with acids like hydrochloric acid or hydrobromic acid which yield the corresponding carbocation intermediate, this carbocation intermediate undergoes substitution reaction which yields the corresponding substitution product.
Tertiary alcohols undergo substitution very fast than the secondary alcohols because tertiary carbocation is more stable than the secondary carbocation than the primary carbocation.
Primary alcohol is less stable therefore it won’t undergo SN1substitution reaction.
SN2 reaction:
The alcohol is reaction with acids like hydrochloric acid or hydrobromic acid, the bromine atom attacks back side of the carbon atoms in simultaneous manner and which is bearing alcohol group which yield the corresponding product.
Example:
Alcohol is reaction with tosyl chloride in pyridine which provides retention of configuration of tosylated compound. This tosylated compound is further reaction with sodium methoxide which undergoes again SN2 type of reaction, the methoxide ion attacks the carbon atom through the back side and provides Inverse configuration of methoxy compound. This is shown below,

SN2 reaction is second order reaction, the rate of the reaction is depending on the both substrate and nucleophiles.
Answer to Problem 45AP
The reaction is given below,
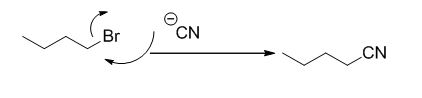
Explanation of Solution
Given information:
The product of the reaction is given below,
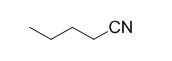
The reaction is given below,
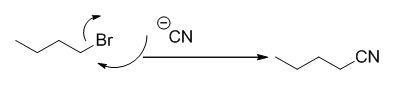
n-butyl bromide undergoes nucleophilic substitution reaction with cyanide (sodium cyanide) gives the corresponding cyanide product.
The given molecule is prepared by using nucleophilic substitution reaction.
d)
Interpretation:
The given molecule has to be prepared by using nucleophilic substitution reaction.
Concept introduction:
SN1 reaction:
The alcohol is reaction with acids like hydrochloric acid or hydrobromic acid which yield the corresponding carbocation intermediate, this carbocation intermediate undergoes substitution reaction which yields the corresponding substitution product.
Tertiary alcohols undergo substitution very fast than the secondary alcohols because tertiary carbocation is more stable than the secondary carbocation than the primary carbocation.
Primary alcohol is less stable therefore it won’t undergo SN1substitution reaction.
SN2 reaction:
The alcohol is reaction with acids like hydrochloric acid or hydrobromic acid, the bromine atom attacks back side of the carbon atoms in simultaneous manner and which is bearing alcohol group which yield the corresponding product.
Example:
Alcohol is reaction with tosyl chloride in pyridine which provides retention of configuration of tosylated compound. This tosylated compound is further reaction with sodium methoxide which undergoes again SN2 type of reaction, the methoxide ion attacks the carbon atom through the back side and provides Inverse configuration of methoxy compound. This is shown below,

SN2 reaction is second order reaction, the rate of the reaction is depending on the both substrate and nucleophiles.
Answer to Problem 45AP
The reaction is given below,
Explanation of Solution
Given information:
The reaction is given below,
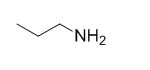
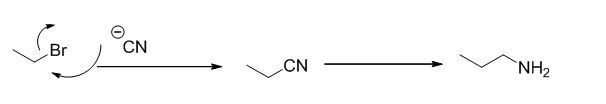
The reaction is given below,
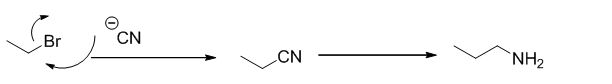
Ethyl bromide undergoes nucleophilic substitution reaction with cyanide (sodium cyanide) gives the corresponding cyanide product, this cyanide undergoes catalytic reduction using Pt (metal reduction) gives
The given molecule is prepared by using nucleophilic substitution reaction.
Want to see more full solutions like this?
Chapter 11 Solutions
EBK ORGANIC CHEMISTRY
- What would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forwardFor benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forward
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

