
EBK FUNDAMENTALS OF GENERAL, ORGANIC, A
8th Edition
ISBN: 8220102895805
Author: Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 11.9, Problem 11.19P
Interpretation Introduction
Interpretation:
The nuclear fission reaction of
Concept Introduction:
Nuclear fission reaction:
The nuclear fission is any a nuclear reaction or a radioactive decay method in which the nucleus of an atom splits into smaller parts.
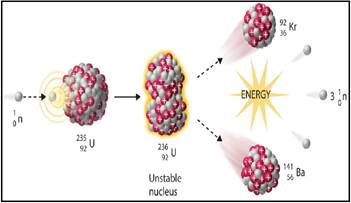
Figure 1
Beta decay:
The
Where the electron
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
how would you make this plot in excel?
what is the product
What is the product?
Chapter 11 Solutions
EBK FUNDAMENTALS OF GENERAL, ORGANIC, A
Ch. 11.4 - Prob. 11.1PCh. 11.4 - Prob. 11.2PCh. 11.4 - Prob. 11.3PCh. 11.4 - Prob. 11.4PCh. 11.4 - Prob. 11.5PCh. 11.4 - Prob. 11.6PCh. 11.4 - Prob. 11.7KCPCh. 11.5 - What are the three main classes of techniques used...Ch. 11.5 - Prob. 11.2CIAPCh. 11.5 - The half-life of carbon-14, an isotope used in...
Ch. 11.5 - Prob. 11.9PCh. 11.5 - Prob. 11.10PCh. 11.5 - Prob. 11.11KCPCh. 11.6 - A -emitting radiation source gives 250 units of...Ch. 11.7 - What is the purpose of food irradiation, and how...Ch. 11.7 - What kind of radiation is used to treat food?Ch. 11.7 - A typical food irradiation application for the...Ch. 11.7 - A solution of selenium-75, a radioisotope used in...Ch. 11.7 - A typical chest X ray exposes a patient to an...Ch. 11.8 - Prob. 11.6CIAPCh. 11.8 - What advantages does MRI have over CT and PET...Ch. 11.8 - Prob. 11.8CIAPCh. 11.8 - Prob. 11.15PCh. 11.8 - The element berkelium, first prepared at the...Ch. 11.8 - Write a balanced nuclear equation for the reaction...Ch. 11.9 - What other isotope besides tellurium-137 is...Ch. 11.9 - Prob. 11.19PCh. 11.9 - One of the possible reactions for nuclear fusion...Ch. 11 - Magnesium-28 decays by emission to give...Ch. 11 - Prob. 11.22UKCCh. 11 - Prob. 11.23UKCCh. 11 - Prob. 11.24UKCCh. 11 - Prob. 11.25UKCCh. 11 - Prob. 11.26UKCCh. 11 - Prob. 11.27UKCCh. 11 - Prob. 11.28UKCCh. 11 - Prob. 11.29UKCCh. 11 - Prob. 11.30APCh. 11 - Describe how radiation, radiation, radiation,...Ch. 11 - Prob. 11.32APCh. 11 - Prob. 11.33APCh. 11 - Prob. 11.34APCh. 11 - Prob. 11.35APCh. 11 - Prob. 11.36APCh. 11 - Prob. 11.37APCh. 11 - Prob. 11.38APCh. 11 - Prob. 11.39APCh. 11 - Prob. 11.40APCh. 11 - Prob. 11.41APCh. 11 - Prob. 11.42APCh. 11 - What characteristic of uranium-235 fission causes...Ch. 11 - What products result from radioactive decay of the...Ch. 11 - Prob. 11.45APCh. 11 - Prob. 11.46APCh. 11 - Prob. 11.47APCh. 11 - Balance the following equations for the nuclear...Ch. 11 - Complete the following nuclear equations and...Ch. 11 - Prob. 11.50APCh. 11 - Cobalt-60 (half-life = 5.3 years) is used to...Ch. 11 - Prob. 11.52APCh. 11 - Prob. 11.53APCh. 11 - Prob. 11.54APCh. 11 - Prob. 11.55APCh. 11 - Selenium-75, a emitter with a half-life of 120...Ch. 11 - Prob. 11.57APCh. 11 - The half-life of mercury-197 is 64.1 hours. If a...Ch. 11 - Gold-198, a emitter used to treat leukemia, has a...Ch. 11 - Describe how a Geiger counter works.Ch. 11 - Prob. 11.61APCh. 11 - Prob. 11.62APCh. 11 - Prob. 11.63APCh. 11 - Prob. 11.64APCh. 11 - Match each unit in the left column with the...Ch. 11 - Prob. 11.66APCh. 11 - Sodium-24 is used to study the circulatory system...Ch. 11 - Prob. 11.68APCh. 11 - Prob. 11.69APCh. 11 - Prob. 11.70CPCh. 11 - Prob. 11.71CPCh. 11 - Prob. 11.72CPCh. 11 - Prob. 11.73CPCh. 11 - Prob. 11.74CPCh. 11 - Prob. 11.75CPCh. 11 - Prob. 11.76CPCh. 11 - Prob. 11.77CPCh. 11 - Prob. 11.78CPCh. 11 - Prob. 11.79CPCh. 11 - Prob. 11.80CPCh. 11 - Prob. 11.81CPCh. 11 - Prob. 11.82CPCh. 11 - Although turning lead into gold in a nuclear...Ch. 11 - Prob. 11.84CPCh. 11 - Prob. 11.85CPCh. 11 - Prob. 11.86CPCh. 11 - Prob. 11.87CPCh. 11 - Prob. 11.88CPCh. 11 - One way to demonstrate the dose factor of ionizing...Ch. 11 - One approach for treating cancerous tumors is...Ch. 11 - Prob. 11.91GP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Problem 15 of 15 Submit Using the following reaction data points, construct Lineweaver-Burk plots for an enzyme with and without an inhibitor by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. Using the information from this plot, determine the type of inhibitor present. 1 mM-1 1 s mM -1 [S]' V' with 10 μg per 20 54 10 36 20 5 27 2.5 23 1.25 20 Answer: |||arrow_forward12:33 CO Problem 4 of 15 4G 54% Done On the following Lineweaver-Burk -1 plot, identify the by dragging the Km point to the appropriate value. 1/V 40 35- 30- 25 20 15 10- T Км -15 10 -5 0 5 ||| 10 15 №20 25 25 30 1/[S] Г powered by desmosarrow_forward1:30 5G 47% Problem 10 of 15 Submit Using the following reaction data points, construct a Lineweaver-Burk plot for an enzyme with and without a competitive inhibitor by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. 1 -1 1 mM [S]' s mM¹ with 10 mg pe 20 V' 54 10 36 > ст 5 27 2.5 23 1.25 20 Answer: |||arrow_forward
- Problem 14 of 15 Submit Using the following reaction data points, construct Lineweaver-Burk plots for an enzyme with and without an inhibitor by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. Using the information from this plot, determine the type of inhibitor present. 1 mM-1 1 s mM -1 [S]' V' with 10 μg per 20 54 10 36 20 5 27 2.5 23 1.25 20 Answer: |||arrow_forward12:36 CO Problem 9 of 15 4G. 53% Submit Using the following reaction data points, construct a Lineweaver-Burk plot by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. Based on the plot, determine the value of the catalytic efficiency (specificity constant) given that the enzyme concentration in this experiment is 5.0 μ.Μ. 1 [S] ¨‚ μM-1 1 V sμM-1 100.0 0.100 75.0 0.080 50.0 0.060 15.0 0.030 10.0 0.025 5.0 0.020 Answer: ||| O Гarrow_forwardProblem 11 of 15 Submit Using the following reaction data points, construct a Lineweaver-Burk plot for an enzyme with and without a noncompetitive inhibitor by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. 1 -1 1 mM [S]' 20 V' s mM¹ with 10 μg per 54 10 36 > ст 5 27 2.5 23 1.25 20 Answer: |||arrow_forward
- Problem 13 of 15 Submit Using the following reaction data points, construct Lineweaver-Burk plots for an enzyme with and without an inhibitor by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. Using the information from this plot, determine the type of inhibitor present. 1 mM-1 1 s mM -1 [S]' V' with 10 μg per 20 54 10 36 20 5 27 2.5 23 1.25 20 Answer: |||arrow_forward12:33 CO Problem 8 of 15 4G. 53% Submit Using the following reaction data points, construct a Lineweaver-Burk plot by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. Based on the plot, determine the value of kcat given that the enzyme concentration in this experiment is 5.0 μM. 1 [S] , мм -1 1 V₁ s μM 1 100.0 0.100 75.0 0.080 50.0 0.060 15.0 0.030 10.0 0.025 5.0 0.020 Answer: ||| Гarrow_forward1:33 5G. 46% Problem 12 of 15 Submit Using the following reaction data points, construct a Lineweaver-Burk plot for an enzyme with and without an uncompetitive inhibitor by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. 1 -1 1 mM [S]' 20 V' s mM¹ with 10 μg per 54 10 36 > ст 5 27 2.5 23 1.25 20 Answer: |||arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage LearningEssentials Health Info Management Principles/Prac...Health & NutritionISBN:9780357191651Author:BowiePublisher:Cengage
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage LearningEssentials Health Info Management Principles/Prac...Health & NutritionISBN:9780357191651Author:BowiePublisher:Cengage

Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning


Essentials Health Info Management Principles/Prac...
Health & Nutrition
ISBN:9780357191651
Author:Bowie
Publisher:Cengage


