
(a)
Interpretation:
To predict the products of the following transesterification reaction.
Concept Introduction:
Transesterification is the process of formation of a new ester molecule from the reaction of alcohol and an ester. This is like hydrolysis of ester but here nucleophile is alcohol molecule instead of
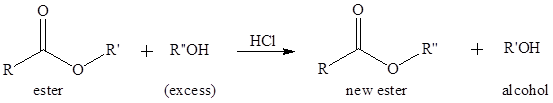
(b)
Interpretation:
To predict the products of the following transesterification reaction.
Concept Introduction:
Transesterification is the process of formation of a new ester molecule from the reaction of alcohol and an ester. This is like hydrolysis of ester but here nucleophile is alcohol molecule instead of
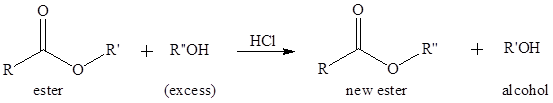
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Essential Organic Chemistry Study Guide & Solution Manual, Books a la Carte Edition
- Nonearrow_forwardNonearrow_forwardIn the solid state, oxalic acid occurs as a dihydrate with the formula H2C2O4 C+2H2O. Use this formula to calculate the formula weight of oxalic acid. Use the calculated formula weight and the number of moles (0.00504mol) of oxalic acid in each titrated unknown sample recorded in Table 6.4 to calculate the number of grams of pure oxalic acid dihydrate contained in each titrated unknown sample.arrow_forward
- 1. Consider a pair of elements with 2p and 4p valence orbitals (e.g., N and Se). Draw their (2p and 4p AO's) radial probability plots, and sketch their angular profiles. Then, consider these orbitals from the two atoms forming a homonuclear л-bond. Which element would have a stronger bond, and why? (4 points)arrow_forwardWrite the reaction and show the mechanism of the reaction. Include the mechanism for formation of the NO2+ 2. Explain, using resonance structures, why the meta isomer is formed. Draw possible resonance structures for ortho, meta and para.arrow_forwardNonearrow_forward
- 3. A molecular form of "dicarbon", C2, can be generated in gas phase. Its bond dissociation energy has been determined at 599 kJ/mol. Use molecular orbital theory to explain why energy of dissociation for C₂+ is 513 kJ/mol, and that for C2² is 818 kJ/mol. (10 points)arrow_forward9.73 g of lead(IV) chloride contains enough Cl- ions to make ____ g of magnesium chloride.arrow_forward6. a) C2's. Phosphorus pentafluoride PF5 belongs to D3h symmetry group. Draw the structure of the molecule, identify principal axis of rotation and perpendicular (4 points) b) assume that the principal axis of rotation is aligned with z axis, assign symmetry labels (such as a1, b2, etc.) to the following atomic orbitals of the P atom. (character table for this group is included in the Supplemental material). 3s 3pz (6 points) 3dz²arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

