
(a)
Interpretation: The reagents that can be used to achieve the following transformation are to be identified.

Concept introduction: The given compound is a terminal
(b)
Interpretation: The reagents that can be used to achieve each of the following transformations are to be identified.

Concept introduction: The starting alkyne is a compound with five carbon atoms, which to be converted into a seven carbon-containing terminal alkyne, needs to undergo a reaction with such reagents which can facilitate this reaction. Reduction using a poisoned catalyst such as Lindlar’s catalyst, followed by bromination and reaction with an alkynide can yield the desired product.
(c)
Interpretation: The reagents that can be used to achieve each of the following transformations are to be identified.

Concept introduction: The starting material has one more carbon atom than the product. This means the synthesis must have an ozonolysis process, to cleave a carbon-carbon bond. Also, since the product is a
(d)
Interpretation: The reagents that can be used to achieve each of the following transformations are to be identified.
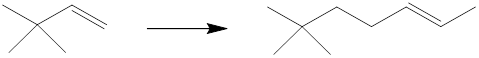
Concept introduction: The starting material has six carbon atoms, and the product has nine carbon atoms. So the synthesis must involve the installation of three carbon atoms and also, the location of the
(e)
Interpretation: The reagents that can be used to achieve each of the following transformations is to be identified.
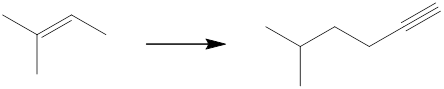
Concept introduction: The product has two more carbon atoms than the starting material, and the location of the functional group has changed. So, bromination followed by dehydrohalogenation can give a terminal alkene, which on further bromination followed by reaction with an alkynide would yield the desired product.
(f)
Interpretation: The reagents that can be used to achieve each of the following transformation is to be identified.
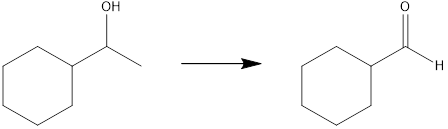
Concept introduction: The starting material has one more carbon atom than the product. Therefore, the synthesis must employ an ozonolysis process, to cleave a carbon-carbon bond. For the formation of an aldehyde product, an alkene is also required. For this alkene to be formed, the alcohol must be converted to a tosylate, and then after the alkene is formed, it can undergo ozonolysis to yield the product.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
ORGANIC CHEM PRINT STUDY GDE & SSM
- #1. Retro-Electrochemical Reaction: A ring has been made, but the light is causing the molecule to un- cyclize. Undo the ring into all possible molecules. (2pts, no partial credit) hvarrow_forwardDon't used Ai solutionarrow_forwardI have a question about this problem involving mechanisms and drawing curved arrows for acids and bases. I know we need to identify the nucleophile and electrophile, but are there different types of reactions? For instance, what about Grignard reagents and other types that I might not be familiar with? Can you help me with this? I want to identify the names of the mechanisms for problems 1-14, such as Gilman reagents and others. Are they all the same? Also, could you rewrite it so I can better understand? The handwriting is pretty cluttered. Additionally, I need to label the nucleophile and electrophile, but my main concern is whether those reactions differ, like the "Brønsted-Lowry acid-base mechanism, Lewis acid-base mechanism, acid-catalyzed mechanisms, acid-catalyzed reactions, base-catalyzed reactions, nucleophilic substitution mechanisms (SN1 and SN2), elimination reactions (E1 and E2), organometallic mechanisms, and so forth."arrow_forward
- 2. 200 LOD For an unknown compound with a molecular ion of 101 m/z: a. Use the molecular ion to propose at least two molecular formulas. (show your work) b. What is the DU for each of your possible formulas? (show your work) C. Solve the structure and assign each of the following spectra. 8 6 4 2 (ppm) 150 100 50 ō (ppm) 4000 3000 2000 1500 1000 500 HAVENUMBERI-11arrow_forwardComplete the spectroscopy with structurearrow_forwardComplete the spectroscopy with structurearrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


