
Chemistry: An Introduction to General, Organic, and Biological Chemistry, Books a la Carte Edition & Modified MasteringChemistry with Pearson eText -- ValuePack Access Card Package
1st Edition
ISBN: 9780133899573
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11.3, Problem 11.14QAP
Give the TUPAC name for each of the following:
a. 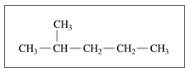
b. 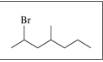
c. 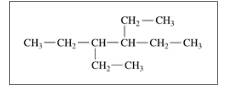
d. 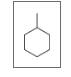
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Part 4. Butanoic acid (ka= 1.52× 10-5) has a partition coefficient of 3.0 (favors benzene) when distributed bet.
water and benzene. What is the formal concentration of butanoic acid in each phase when
0.10M aqueous butanoic acid is extracted w❘ 25 mL of benzene
100 mL of
a) at pit 5.00
b) at pH 9.00
Calculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 Group of answer choices 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/mole
Calculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 choices: 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/mole
Chapter 11 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry, Books a la Carte Edition & Modified MasteringChemistry with Pearson eText -- ValuePack Access Card Package
Ch. 11.1 - Prob. 11.1QAPCh. 11.1 - Prob. 11.2QAPCh. 11.1 - Identify each of the following properties as more...Ch. 11.1 - Identify each of the following properties as more...Ch. 11.1 - Prob. 11.5QAPCh. 11.1 - Prob. 11.6QAPCh. 11.2 - Give the IUPAC name for each of the following...Ch. 11.2 - Give the IUPAC name for each of the following...Ch. 11.2 - Prob. 11.9QAPCh. 11.2 - Draw the condensed structural formula for alkanes...
Ch. 11.3 - Indicate whether each of the following pairs...Ch. 11.3 - Indicate whether each of the following pairs...Ch. 11.3 - Give the IUPAC name for each of the following a....Ch. 11.3 - Give the TUPAC name for each of the following: a....Ch. 11.3 - Draw the condensed structural formula for each of...Ch. 11.3 - Draw the condensed structural formula for each of...Ch. 11.3 - Draw the line-angle formula for each of the...Ch. 11.3 - Prob. 11.18QAPCh. 11.4 - Heptane, used as a solvent for rubber cement, has...Ch. 11.4 - Nonane has a density of 0.79 g/mL and boils at 151...Ch. 11.4 - Prob. 11.21QAPCh. 11.4 - Prob. 11.22QAPCh. 11.5 - Prob. 11.23QAPCh. 11.5 - Identify the following as alkanes, alkenes,...Ch. 11.5 - Give the IUPAC name for each of the following: a....Ch. 11.5 - Give the IUPAC name for each of the following: a....Ch. 11.5 - Draw the condensed structural formula, or...Ch. 11.5 - Prob. 11.28QAPCh. 11.6 - Prob. 11.29QAPCh. 11.6 - Prob. 11.30QAPCh. 11.6 - Prob. 11.31QAPCh. 11.6 - Prob. 11.32QAPCh. 11.7 - Prob. 11.33QAPCh. 11.7 - Prob. 11.34QAPCh. 11.8 - Prob. 11.35QAPCh. 11.8 - Prob. 11.36QAPCh. 11.8 - Prob. 11.37QAPCh. 11.8 - Prob. 11.38QAPCh. 11 - Prob. 11.39UTCCh. 11 - Prob. 11.40UTCCh. 11 - Prob. 11.41UTCCh. 11 - Prob. 11.42UTCCh. 11 - Prob. 11.43UTCCh. 11 - Convert each of the following line-angle formulas...Ch. 11 - Give the IUPAC name for each of the following:...Ch. 11 - Give the IUPAC name for each of the following:...Ch. 11 - Give the IUPAC name (including cis or trans, if...Ch. 11 - Give the LUPAC name (including cis or trans, if...Ch. 11 - Prob. 11.49AQAPCh. 11 - Prob. 11.50AQAPCh. 11 - Prob. 11.51AQAPCh. 11 - Prob. 11.52AQAPCh. 11 - Draw the condensed structural or line-angle...Ch. 11 - Draw the condensed structural or line-angle...Ch. 11 - Draw the cis and trans isomers for each of the...Ch. 11 - Draw the cis and trans isomers for each of the...Ch. 11 - Prob. 11.57AQAPCh. 11 - Prob. 11.58AQAPCh. 11 - Prob. 11.59AQAPCh. 11 - Prob. 11.60AQAPCh. 11 - Prob. 11.61AQAPCh. 11 - Give the name for the product from the...Ch. 11 - Prob. 11.63AQAPCh. 11 - Prob. 11.64AQAPCh. 11 - Prob. 11.65CQCh. 11 - Prob. 11.66CQCh. 11 - Prob. 11.67CQCh. 11 - Prob. 11.68CQCh. 11 - Prob. 11.69CQCh. 11 - Prob. 11.70CQCh. 11 - Prob. 11.71CQCh. 11 - Margarines are produced from the hydrogenation of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the rate law for sodium thiosulfate from the following data: [Na2S2O3] Time (s) 0.0318 230. 0.0636 57.5arrow_forwardPlease solvearrow_forwardRank the compounds in each group below according to their reactivity toward electrophilic aromatic substitution (most reactive = 1; least reactive = 3). Place the number corresponding to the compounds' relative reactivity in the blank below the compound. a. CH₂F CH3 F b. At what position, and on what ring, is bromination of phenyl benzoate expected to occur? Explain your answer. :0: C-O phenyl benzoate 6.Consider the reaction below to answer the following questions. A B C NO₂ FeBr3 + Br₂ D a. The nucleophile in the reaction is: BODADES b. The Lewis acid catalyst in the reaction is: C. This reaction proceeds d. Draw the structure of product D. (faster or slower) than benzene.arrow_forward
- Part 2. A solution of 6.00g of substance B in 100.0mL of aqueous solution is in equilibrium, at room temperature, wl a solution of B in diethyl ether (ethoxyethane) containing 25.0 g of B in 50.0 mL 9) what is the distribution coefficient of substance B b) what is the mass of B extracted by shaking 200 ml of an aqueous solution containing 10g of B with call at room temp): i) 100 mL of diethyl ether ii) 50ml of diethyl ether twice iii) 25ml of diethyl ether four timesarrow_forward- Rank the following groups of compounds from most acidic (1) to least acidic (4). Place the number corresponding to the compound's relative rank in the blank below the structure. a. NO₂ NO₂ CH2CH2CH2CH2OH CH3 CH3CH2CHOH CH3CH2CH2CH2OH NO₂ CH3CHCH2CH2OH b. OH OH CH₂OH CO₂H HC CN CN CNarrow_forwardGive the major organic product(s) of the following reactions or sequences of reactions. Show all relevant stereochemistry a. H MgBr 1. ether 2. H₂O* 4 COH b. 1. LIAIH, ether 2. H₂O Choose the best reagent(s) for carrying out the following conversions from the list provided below. Place the letter of the best choice in the blank to the left of the conversion. Reagents may be used more than once. a. 1. CH3MgBr, ether 2. H3O+ NaOH b. 1. PBr3 2. C. 2. 1. (CH3)3SiCl, (CH3CH2)3N CH3MgBr, ether 3. H₂O*+ 2. H3O+ e. 1. p-TosCl, pyridine f. نها g. 2. NaOH CrO3, H₂SO4, H₂O 1. NaBH4, ethanol 2. H30* h. PCC, CH2Cl2 Ovoldo-6 a. b. OH OH H OH O any organicarrow_forward
- Determine the rate law for sodium thiosulfate from the following data: [Na2S2O3] Time (s) 0.0318 230. 0.0636 57.5arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardDetermine the rate order from the following data: [Na2S2O3] Time (s) 0.0455 M 180.0 0.0909 M 90.0 0.136 M 60.0 Group of answer choices First order Second order Third order Zero orderarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY