
Chemistry: An Introduction to General, Organic, and Biological Chemistry, Books a la Carte Edition & Modified MasteringChemistry with Pearson eText -- ValuePack Access Card Package
1st Edition
ISBN: 9780133899573
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11.3, Problem 11.11QAP
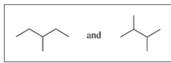
Indicate whether each of the following pairs represent structural isomers or the same molecule:
a.
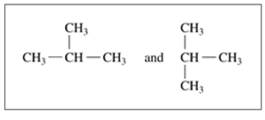
b.
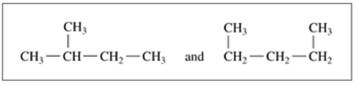
C
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Could you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!
Could you please solve the first problem in this way and present it similarly but (color-coded) and step by step so I can understand it better? Thank you! I want to see what they are doing
Can you please help mne with this problem. Im a visual person, so can you redraw it, potentislly color code and then as well explain it. I know im given CO2 use that to explain to me, as well as maybe give me a second example just to clarify even more with drawings (visuals) and explanations.
Chapter 11 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry, Books a la Carte Edition & Modified MasteringChemistry with Pearson eText -- ValuePack Access Card Package
Ch. 11.1 - Prob. 11.1QAPCh. 11.1 - Prob. 11.2QAPCh. 11.1 - Identify each of the following properties as more...Ch. 11.1 - Identify each of the following properties as more...Ch. 11.1 - Prob. 11.5QAPCh. 11.1 - Prob. 11.6QAPCh. 11.2 - Give the IUPAC name for each of the following...Ch. 11.2 - Give the IUPAC name for each of the following...Ch. 11.2 - Prob. 11.9QAPCh. 11.2 - Draw the condensed structural formula for alkanes...
Ch. 11.3 - Indicate whether each of the following pairs...Ch. 11.3 - Indicate whether each of the following pairs...Ch. 11.3 - Give the IUPAC name for each of the following a....Ch. 11.3 - Give the TUPAC name for each of the following: a....Ch. 11.3 - Draw the condensed structural formula for each of...Ch. 11.3 - Draw the condensed structural formula for each of...Ch. 11.3 - Draw the line-angle formula for each of the...Ch. 11.3 - Prob. 11.18QAPCh. 11.4 - Heptane, used as a solvent for rubber cement, has...Ch. 11.4 - Nonane has a density of 0.79 g/mL and boils at 151...Ch. 11.4 - Prob. 11.21QAPCh. 11.4 - Prob. 11.22QAPCh. 11.5 - Prob. 11.23QAPCh. 11.5 - Identify the following as alkanes, alkenes,...Ch. 11.5 - Give the IUPAC name for each of the following: a....Ch. 11.5 - Give the IUPAC name for each of the following: a....Ch. 11.5 - Draw the condensed structural formula, or...Ch. 11.5 - Prob. 11.28QAPCh. 11.6 - Prob. 11.29QAPCh. 11.6 - Prob. 11.30QAPCh. 11.6 - Prob. 11.31QAPCh. 11.6 - Prob. 11.32QAPCh. 11.7 - Prob. 11.33QAPCh. 11.7 - Prob. 11.34QAPCh. 11.8 - Prob. 11.35QAPCh. 11.8 - Prob. 11.36QAPCh. 11.8 - Prob. 11.37QAPCh. 11.8 - Prob. 11.38QAPCh. 11 - Prob. 11.39UTCCh. 11 - Prob. 11.40UTCCh. 11 - Prob. 11.41UTCCh. 11 - Prob. 11.42UTCCh. 11 - Prob. 11.43UTCCh. 11 - Convert each of the following line-angle formulas...Ch. 11 - Give the IUPAC name for each of the following:...Ch. 11 - Give the IUPAC name for each of the following:...Ch. 11 - Give the IUPAC name (including cis or trans, if...Ch. 11 - Give the LUPAC name (including cis or trans, if...Ch. 11 - Prob. 11.49AQAPCh. 11 - Prob. 11.50AQAPCh. 11 - Prob. 11.51AQAPCh. 11 - Prob. 11.52AQAPCh. 11 - Draw the condensed structural or line-angle...Ch. 11 - Draw the condensed structural or line-angle...Ch. 11 - Draw the cis and trans isomers for each of the...Ch. 11 - Draw the cis and trans isomers for each of the...Ch. 11 - Prob. 11.57AQAPCh. 11 - Prob. 11.58AQAPCh. 11 - Prob. 11.59AQAPCh. 11 - Prob. 11.60AQAPCh. 11 - Prob. 11.61AQAPCh. 11 - Give the name for the product from the...Ch. 11 - Prob. 11.63AQAPCh. 11 - Prob. 11.64AQAPCh. 11 - Prob. 11.65CQCh. 11 - Prob. 11.66CQCh. 11 - Prob. 11.67CQCh. 11 - Prob. 11.68CQCh. 11 - Prob. 11.69CQCh. 11 - Prob. 11.70CQCh. 11 - Prob. 11.71CQCh. 11 - Margarines are produced from the hydrogenation of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part 1. Aqueous 0.010M AgNO 3 is slowly added to a 50-ml solution containing both carbonate [co32-] = 0.105 M and sulfate [soy] = 0.164 M anions. Given the ksp of Ag2CO3 and Ag₂ soy below. Answer the ff: Ag₂ CO3 = 2 Ag+ caq) + co} (aq) ksp = 8.10 × 10-12 Ag₂SO4 = 2Ag+(aq) + soy² (aq) ksp = 1.20 × 10-5 a) which salt will precipitate first? (b) What % of the first anion precipitated will remain in the solution. by the time the second anion starts to precipitate? (c) What is the effect of low pH (more acidic) condition on the separate of the carbonate and sulfate anions via silver precipitation? What is the effect of high pH (more basic)? Provide appropriate explanation per answerarrow_forwardPart 4. Butanoic acid (ka= 1.52× 10-5) has a partition coefficient of 3.0 (favors benzene) when distributed bet. water and benzene. What is the formal concentration of butanoic acid in each phase when 0.10M aqueous butanoic acid is extracted w❘ 25 mL of benzene 100 mL of a) at pit 5.00 b) at pH 9.00arrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 Group of answer choices 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forward
- Calculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 choices: 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Rank the compounds in each group below according to their reactivity toward electrophilic aromatic substitution (most reactive = 1; least reactive = 3). Place the number corresponding to the compounds' relative reactivity in the blank below the compound. a. CH₂F CH3 F b. At what position, and on what ring, is bromination of phenyl benzoate expected to occur? Explain your answer. :0: C-O phenyl benzoate 6.Consider the reaction below to answer the following questions. A B C NO₂ FeBr3 + Br₂ D a. The nucleophile in the reaction is: BODADES b. The Lewis acid catalyst in the reaction is: C. This reaction proceeds d. Draw the structure of product D. (faster or slower) than benzene.arrow_forwardPart 2. A solution of 6.00g of substance B in 100.0mL of aqueous solution is in equilibrium, at room temperature, wl a solution of B in diethyl ether (ethoxyethane) containing 25.0 g of B in 50.0 mL 9) what is the distribution coefficient of substance B b) what is the mass of B extracted by shaking 200 ml of an aqueous solution containing 10g of B with call at room temp): i) 100 mL of diethyl ether ii) 50ml of diethyl ether twice iii) 25ml of diethyl ether four timesarrow_forward- Rank the following groups of compounds from most acidic (1) to least acidic (4). Place the number corresponding to the compound's relative rank in the blank below the structure. a. NO₂ NO₂ CH2CH2CH2CH2OH CH3 CH3CH2CHOH CH3CH2CH2CH2OH NO₂ CH3CHCH2CH2OH b. OH OH CH₂OH CO₂H HC CN CN CNarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY