
A refrigerator uses refrigerant-134a as the working fluid and operates on the vapor-compression refrigeration cycle. The evaporator and condenser pressures are 200 kPa and 1400 kPa, respectively. The isentropic efficiency of the compressor is 88 percent. The refrigerant enters the compressor at a rate of 0.025 kg/s superheated by 10.1°C and leaves the condenser subcooled by 4.4°C. Determine (a) the rate of cooling provided by the evaporator, the power input, and the COP. Determine (b) the same parameters if the cycle operated on the ideal vapor-compression refrigeration cycle between the same pressure limits.
(a)
The rate of cooling provided by the evaporator, the power input and the COP.
Answer to Problem 21P
The rate of cooling provided by the evaporator is
Explanation of Solution
Show the T-s diagram for the vapor-compression refrigeration cycle as in Figure (1).
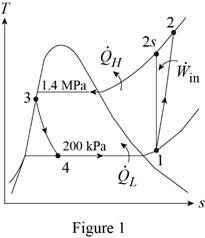
Express specific enthalpy at state 2 by using the formula of Carnot efficiency.
Here, Carnot efficiency is
Express rate of heat lost by the evaporator.
Here, mass flow rate of refrigerant is
Express rate of heat supplied to the evaporator.
Here, specific enthalpy at state 3 is
Express power input.
Express coefficient of performance.
Express initial temperature.
Here, saturated temperature at initial pressure of
Express temperature at state 3.
Here, saturated temperature at pressure at state 3 of
Conclusion:
Refer Table A-12, “saturated refrigerant 134a-pressure table”, and write the saturated temperature at initial pressure of
Substitute
Refer Table A-12, “saturated refrigerant 134a-pressure table”, and write the saturated temperature at pressure at state 3 of
Substitute
Refer Table A-13, “superheated refrigerant-134a”, and write the properties corresponding to initial pressure
Here, initial specific entropy is
From Figure (1), the initial specific entropy is equal to specific entropy at state 2.
Perform unit conversion of pressure at state 2 from
Refer Table A-13, “superheated refrigerant 134a”, and write the specific enthalpy at state 2s corresponding to pressure at state 2 of
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y is specific entropy at state 2 and specific enthalpy at state 2 respectively.
Show the specific enthalpy at state 2s corresponding to specific entropy as in Table (1).
|
Specific entropy at state 2 |
Specific enthalpy at state 2s |
| 0.9389 | 285.47 |
| 0.9699 | |
| 0.9733 | 297.10 |
Substitute
From Figure (1), write the specific enthalpy at state 3 is equal to state 4 due to throttling process.
Here, specific enthalpy at state 3 is
Refer Table A-11, “saturated refrigerant-134a-pressure table”, and write the specific enthalpy at state 3 corresponding to temperature at state 3 of
Substitute
Substitute
Substitute
Hence, the rate of cooling provided by the evaporator is
Substitute
Hence, the power input is
Substitute
Hence, the COP is
(b)
The rate of cooling provided by the evaporator, the power input and the COP.
Answer to Problem 21P
The rate of cooling provided by the evaporator is
Explanation of Solution
Show the T-s diagram for the ideal vapor-compression refrigeration cycle as in Figure (2).
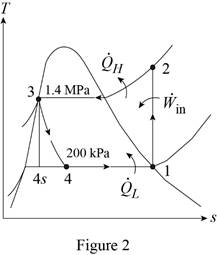
Express rate of heat lost by the evaporator.
Here, mass flow rate of refrigerant is
Express rate of heat supplied to the evaporator.
Here, specific enthalpy at state 3 is
Express power input.
Express coefficient of performance.
Conclusion:
From Figure (2), write the specific enthalpy at state 3 is equal to state 4 due to throttling process.
Here, specific enthalpy at state 3 is
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the properties corresponding to pressure at state 1
Here, specific entropy at state 1 is
Refer Table A-13, “superheated refrigerant 134a”, and write the specific enthalpy at state 2 corresponding to pressure at state 2 of
Show the specific enthalpy at state 2 corresponding to specific entropy as in Table (2).
|
Specific entropy at state 2 |
Specific enthalpy at state 2 |
| 0.9107 | 276.17 |
| 0.9378 | |
| 0.9389 | 285.47 |
Substitute
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the specific enthalpy at state 3 corresponding to pressure at state 3
Here, specific enthalpy at saturated liquid is
Substitute
Substitute
Hence, the rate of cooling provided by the evaporator is
Substitute
Hence, the power input is
Substitute
Hence, the COP is
Want to see more full solutions like this?
Chapter 11 Solutions
THERMODYNAMICS: ENG APPROACH LOOSELEAF
- My ID#016948724 please solve this problems and show me every step clear to follow pleasearrow_forwardMy ID# 016948724arrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forward
- Please do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forward[Q2]: The cost information supplied by the cost accountant is as follows:Sales 20,00 units, $ 10 per unitCalculate the (a/ newsale guantity and (b) new selling price to earn the sameVariable cost $ 6 per unit, Fixed Cost $ 30,000, Profit $ 50,000profit ifi) Variable cost increases by $ 2 per unitil) Fixed cost increase by $ 10,000Ili) Variable cost increase by $ 1 per unit and fixed cost reduces by $ 10,000arrow_forward
- can you please help me perform Visual Inspection and Fractography of the attatched image: Preliminary examination to identify the fracture origin, suspected fatigue striation, and corrosion evidences.arrow_forwardcan you please help[ me conduct Causal Analysis (FTA) on the scenario attatched: FTA diagram which is a fault tree analysis diagram will be used to gain an overview of the entire path of failure from root cause to the top event (i.e., the swing’s detachment) and to identify interactions between misuse, material decay and inspection errors.arrow_forwardhi can you please help me in finding the stress intensity factor using a k-calcluator for the scenario attathced in the images.arrow_forward
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
