
EBK THERMODYNAMICS: AN ENGINEERING APPR
9th Edition
ISBN: 8220106796979
Author: CENGEL
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.11, Problem 58P
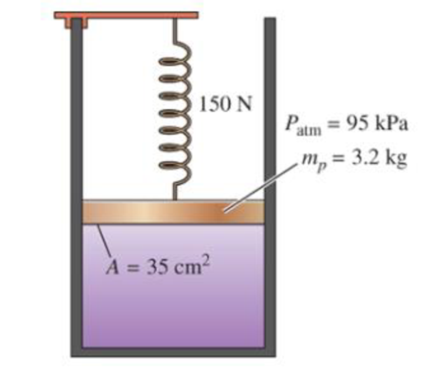
A gas is contained in a vertical, frictionless piston–cylinder device. The piston has a mass of 3.2 kg and a cross-sectional area of 35 cm2. A compressed spring above the piston exerts a force of 150 N on the piston. If the atmospheric pressure is 95 kPa, determine the pressure inside the cylinder. Answer: 147 kPa
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The gears shown in the figure have a diametral pitch of 2 teeth per inch and a 20° pressure angle.
The pinion rotates at 1800 rev/min clockwise and transmits 200 hp through the idler pair to gear
5 on shaft c. What forces do gears 3 and 4 transmit to the idler shaft?
TS
I
y
18T
32T
This
a
12
x
18T
C
48T
5
Question 1. Draw 3 teeth for the following pinion and gear respectively. The teeth
should be drawn near the pressure line so that the teeth from the pinion should
mesh those of the gear. Drawing scale (1:1). Either a precise hand drawing or
CAD drawing is acceptable. Draw all the trajectories of the involute lines and the
circles.
Specification: 18tooth pinion and 30tooth gear. Diameter pitch=P=6 teeth /inch.
Pressure angle:20°, 1/P for addendum (a) and 1.25/P for dedendum (b). For fillet,
c=b-a.
5. The figure shows a gear train. There is no friction at the bearings except for the gear tooth forces.
The material of the milled gears is steel having a Brinell hardness of 170. The input shaft speed (n2)
is 800 rpm. The face width and the contact angle for all gears are 1 in and 20° respectively. In this
gear set, the endurance limit (Se) is 15 kpsi and nd (design factor) is 2.
(a) Find the revolution speed of gear 5.
(b) Determine whether each gear satisfies the design factor of 2.0 for bending fatigue.
(c) Determine whether each gear satisfies the design factor of 2.0 for surface fatigue (contact stress).
(d) According to the computation results of the questions (b) and (c), explain the possible failure
mechanisms for each gear.
N4=28
800rpm
N₁=43
N5=34
N₂=14
P(diameteral pitch)=8 for all gears
Coupled to 2.5hp motor
Chapter 1 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
Ch. 1.11 - The value of the gravitational acceleration g...Ch. 1.11 - One of the most amusing things a person can...Ch. 1.11 - An office worker claims that a cup of cold coffee...Ch. 1.11 - What is the difference between the classical and...Ch. 1.11 - Explain why the light-year has the dimension of...Ch. 1.11 - What is the difference between pound-mass and...Ch. 1.11 - What is the net force acting on a car cruising at...Ch. 1.11 - What is the weight, in N, of an object with a mass...Ch. 1.11 - If the mass of an object is 10 lbm, what is its...Ch. 1.11 - The acceleration of high-speed aircraft is...
Ch. 1.11 - The value of the gravitational acceleration g...Ch. 1.11 - A 3-kg plastic tank that has a volume of 0.2 m3 is...Ch. 1.11 - A 2-kg rock is thrown upward with a force of 200 N...Ch. 1.11 - Solve Prob. 113 using appropriate software. Print...Ch. 1.11 - A 4-kW resistance heater in a water heater runs...Ch. 1.11 - A 150-lbm astronaut took his bathroom scale (a...Ch. 1.11 - The gas tank of a car is filled with a nozzle that...Ch. 1.11 - How would you define a system to determine the...Ch. 1.11 - A large fraction of the thermal energy generated...Ch. 1.11 - A can of soft drink at room temperature is put...Ch. 1.11 - How would you define a system to determine the...Ch. 1.11 - How would you describe the state of the air in the...Ch. 1.11 - What is the difference between intensive and...Ch. 1.11 - The specific weight of a system is defined as the...Ch. 1.11 - Is the number of moles of a substance contained in...Ch. 1.11 - Is the state of the air in an isolated room...Ch. 1.11 - What is a quasi-equilibrium process? What is its...Ch. 1.11 - Define the isothermal, isobaric, and isochoric...Ch. 1.11 - What is specific gravity? How is it related to...Ch. 1.11 - What are the ordinary and absolute temperature...Ch. 1.11 - Consider an alcohol and a mercury thermometer that...Ch. 1.11 - Consider two dosed systems A and B. System A...Ch. 1.11 - Consider a system whose temperature is 18C....Ch. 1.11 - Steam enters a heat exchanger at 300 K. What is...Ch. 1.11 - The temperature of a system rises by 130C during a...Ch. 1.11 - The temperature of a system drops by 45F during a...Ch. 1.11 - The temperature of the lubricating oil in an...Ch. 1.11 - Heated air is at 150C. What is the temperature of...Ch. 1.11 - What is the difference between gage pressure and...Ch. 1.11 - Explain why some people experience nose bleeding...Ch. 1.11 - A health magazine reported that physicians...Ch. 1.11 - Someone claims that the absolute pressure in a...Ch. 1.11 - Consider two identical fans, one at sea level and...Ch. 1.11 - The absolute pressure in a compressed air tank is...Ch. 1.11 - A manometer measures a pressure difference as 40...Ch. 1.11 - A vacuum gage connected to a chambee reads 35 kPa...Ch. 1.11 - The maximum safe air pressure of a tire is...Ch. 1.11 - A pressure gage connected to a tank reads 50 psi...Ch. 1.11 - A pressure gage connected to a tank reads 500 kPa...Ch. 1.11 - A 200-pound man has a total foot imprint area of...Ch. 1.11 - The gage pressure in a liquid at a depth of 3 m is...Ch. 1.11 - The absolute pressure in water at a depth of 9 m...Ch. 1.11 - Consider a 1.75-m-tall man standing vertically in...Ch. 1.11 - The barometer of a mountain hiker reads 750 mbars...Ch. 1.11 - The basic barometer can be used to measure the...Ch. 1.11 - A gas is contained in a vertical, frictionless...Ch. 1.11 - Reconsider Prob. 158. Using appropriate software,...Ch. 1.11 - The piston of a vertical piston-cylinder device...Ch. 1.11 - Both a gage and a manometer are attached to a gas...Ch. 1.11 - Reconsider Prob. 161. Using appropriate software,...Ch. 1.11 - A manometer containing oil ( = 850 kg/m3) is...Ch. 1.11 - A manometer is used to measure the air pressure in...Ch. 1.11 - A mercury manometer ( = 13.600 kg/m3) is connected...Ch. 1.11 - Repeat Prob. 165 for a differential mercury height...Ch. 1.11 - The pressure in a natural gas pipeline is measured...Ch. 1.11 - Repeat Prob. 167E by replacing air with oil with a...Ch. 1.11 - Blood pressure is usually measure by wrapping a...Ch. 1.11 - The maximum blood pressure in the upper arm of a...Ch. 1.11 - Consider a U-tube whose arms are open to the...Ch. 1.11 - Consider a double-fluid manometer attached to an...Ch. 1.11 - Calculate the absolute pressure. P1, of the...Ch. 1.11 - Consider the manometer in Fig. 173. If the...Ch. 1.11 - Consider the manometer in Fig. 173. If the...Ch. 1.11 - The hydraulic lift in a car repair shop has an...Ch. 1.11 - Consider the system shown in Fig. 177. If a change...Ch. 1.11 - The gage pressure of the air in the tank shown in...Ch. 1.11 - Repeat Prob. 178 for a gage pressure of 40 kPa.Ch. 1.11 - What is the value of the engineering software...Ch. 1.11 - Determine a positive real root of this equation...Ch. 1.11 - Solve this system of two equations with two...Ch. 1.11 - Solve this system of three equations with three...Ch. 1.11 - Solve this system of three equations with three...Ch. 1.11 - The reactive force developed by a jet engine to...Ch. 1.11 - The reactive force developed by a jet engine to...Ch. 1.11 - A man goes to a traditional market to buy a steak...Ch. 1.11 - What is the weight of a 1-kg substance in N, kN,...Ch. 1.11 - The pressure in a steam boiler is given to be 92...Ch. 1.11 - A hydraulic lift is to be used to lift a 1900-kg...Ch. 1.11 - The average atmosphere pressure on earth is...Ch. 1.11 - Hyperthermia of 5C (i.e., 5C rise above the normal...Ch. 1.11 - The boiling temperature of water decreases by...Ch. 1.11 - A house is losing heat at a rate of 1800 kJ/h per...Ch. 1.11 - The average body temperature of a person rises by...Ch. 1.11 - The average temperature of the atmosphere in the...Ch. 1.11 - A vertical, frictionless pistoncylinder device...Ch. 1.11 - A vertical pistoncylinder device contains a gas at...Ch. 1.11 - The force generated by a spring is given by F =...Ch. 1.11 - An air-conditioning system requires a 35-m-long...Ch. 1.11 - Balloons are often filled with helium gas because...Ch. 1.11 - Reconsider Prob. 1101. Using appropriate software,...Ch. 1.11 - Determine the maximum amount of load, in kg, the...Ch. 1.11 - The lower half of a 6-m-high cylindrical container...Ch. 1.11 - A pressure cooker cooks a lot faster than an...Ch. 1.11 - The pilot of an airplane reads the altitude 6400 m...Ch. 1.11 - A glass tube is attached to a water pipe, as shown...Ch. 1.11 - Consider a U-tube whose arms are open to the...Ch. 1.11 - A water pipe is connected to a double-U manometer...Ch. 1.11 - A gasoline line is connected to a pressure gage...Ch. 1.11 - Repeat Prob. 1110 for a pressure gage reading of...Ch. 1.11 - When measuring small pressure differences with a...Ch. 1.11 - Pressure transducers are commonly used to measure...Ch. 1.11 - Consider the flow of air through a wind turbine...Ch. 1.11 - The drag force exerted on a car by air depends on...Ch. 1.11 - It is well known that cold air feels much colder...Ch. 1.11 - Reconsider Prob. 1116E. Using appropriate...Ch. 1.11 - During a heating process, the temperature of an...Ch. 1.11 - An apple loses 3.6 kJ of heat as it cools per C...Ch. 1.11 - At sea level, the weight of 1 kg mass in SI units...Ch. 1.11 - Consider a fish swimming 5 m below the free...Ch. 1.11 - The atmospheric pressures at the top and the...Ch. 1.11 - Consider a 2.5-m-deep swimming pool. The pressure...
Additional Engineering Textbook Solutions
Find more solutions based on key concepts
What is an uninitialized variable?
Starting Out with Programming Logic and Design (5th Edition) (What's New in Computer Science)
The following C++ program will not compile because the lines have been mixed up. cout Success\n; cout Success...
Starting Out with C++ from Control Structures to Objects (9th Edition)
How are relationships between tables expressed in a relational database?
Modern Database Management
Assume a telephone signal travels through a cable at two-thirds the speed of light. How long does it take the s...
Electric Circuits. (11th Edition)
Why is the study of database technology important?
Database Concepts (8th Edition)
What types of coolant are used in vehicles?
Automotive Technology: Principles, Diagnosis, And Service (6th Edition) (halderman Automotive Series)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 1. The rotating steel shaft is simply supported by bearings at points of B and C, and is driven by a spur gear at D, which has a 6-in pitch diameter. The force F from the drive gear acts at a pressure angle of 20°. The shaft transmits a torque to point A of TA =3000 lbĘ in. The shaft is machined from steel with Sy=60kpsi and Sut=80 kpsi. (1) Draw a shear force diagram and a bending moment diagram by F. According to your analysis, where is the point of interest to evaluate the safety factor among A, B, C, and D? Describe the reason. (Hint: To find F, the torque Tд is generated by the tangential force of F (i.e. Ftangential-Fcos20°) When n=2.5, K=1.8, and K₁ =1.3, determine the diameter of the shaft based on (2) static analysis using DE theory (note that fatigue stress concentration factors need to be used for this question because the loading condition is fatigue) and (3) a fatigue analysis using modified Goodman. Note) A standard diameter is not required for the questions. 10 in Darrow_forward3 N2=28 P(diametral pitch)=8 for all gears Coupled to 25 hp motor N3=34 Full depth spur gears with pressure angle=20° N₂=2000 rpm (1) Compute the circular pitch, the center-to-center distance, and base circle radii. (2) Draw the free body diagram of gear 3 and show all the forces and the torque. (3) In mounting gears, the center-to-center distance was reduced by 0.1 inch. Calculate the new values of center-to-center distance, pressure angle, base circle radii, and pitch circle diameters. (4)What is the new tangential and radial forces for gear 3? (5) Under the new center to center distance, is the contact ratio (mc) increasing or decreasing?arrow_forward2. A flat belt drive consists of two 4-ft diameter cast-iron pulleys spaced 16 ft apart. A power of 60 hp is transmitted by a pulley whose speed is 380 rev/min. Use a service factor (Ks) pf 1.1 and a design factor 1.0. The width of the polyamide A-3 belt is 6 in. Use CD=1. Answer the following questions. (1) What is the total length of the belt according to the given geometry? (2) Find the centrifugal force (Fc) applied to the belt. (3) What is the transmitted torque through the pulley system given 60hp? (4) Using the allowable tension, find the force (F₁) on the tight side. What is the tension at the loose side (F2) and the initial tension (F.)? (5) Using the forces, estimate the developed friction coefficient (f) (6) Based on the forces and the given rotational speed, rate the pulley set. In other words, what is the horse power that can be transmitted by the pulley system? (7) To reduce the applied tension on the tight side, the friction coefficient is increased to 0.75. Find out the…arrow_forward
- The tooth numbers for the gear train illustrated are N₂ = 24, N3 = 18, №4 = 30, №6 = 36, and N₁ = 54. Gear 7 is fixed. If shaft b is turned through 5 revolutions, how many turns will shaft a make? a 5 [6] barrow_forwardCE-112 please solve this problem step by step and give me the correct answerarrow_forwardCE-112 please solve this problem step by step and give me the correct answerarrow_forward
- CE-112 solve this problem step by step and give me the correct answer pleasearrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 International Edition---engineering Mechanics: St...Mechanical EngineeringISBN:9781305501607Author:Andrew Pytel And Jaan KiusalaasPublisher:CENGAGE L
International Edition---engineering Mechanics: St...Mechanical EngineeringISBN:9781305501607Author:Andrew Pytel And Jaan KiusalaasPublisher:CENGAGE L Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning

International Edition---engineering Mechanics: St...
Mechanical Engineering
ISBN:9781305501607
Author:Andrew Pytel And Jaan Kiusalaas
Publisher:CENGAGE L

Refrigeration and Air Conditioning Technology (Mi...
Mechanical Engineering
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:Cengage Learning

Principles of Heat Transfer (Activate Learning wi...
Mechanical Engineering
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Cengage Learning
Thermodynamics: Maxwell relations proofs 1 (from ; Author: lseinjr1;https://www.youtube.com/watch?v=MNusZ2C3VFw;License: Standard Youtube License