
ORGANIC CHEMISTRY-PRINT (LL)-W/WILEY
4th Edition
ISBN: 9781119761105
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 1.10, Problem 23CC
Interpretation Introduction
Interpretation: The indicated bonds should be ranked in increasing bond length:
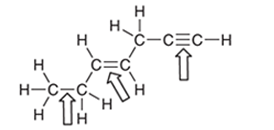
Concept Introduction: The average of the distance between nuclei of two atoms that are covalently bonded in a molecule is said to be bond length. The number of bonded electrons determines the length of the bond, if the number of bonded electrons are higher than the attraction between the two atoms is stronger resulting in shorter bond length.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the most likely mechanism for the following
d.
'Ph
Draw the most likely mechanism for the following
Chapter 1 Solutions
ORGANIC CHEMISTRY-PRINT (LL)-W/WILEY
Ch. 1.2 - Prob. 1LTSCh. 1.2 - Prob. 2ATSCh. 1.2 - Prob. 2LTSCh. 1.3 - Prob. 3LTSCh. 1.3 - Prob. 4PTSCh. 1.3 - Prob. 5PTSCh. 1.4 - Prob. 4LTSCh. 1.4 - Prob. 7PTSCh. 1.4 - Prob. 8PTSCh. 1.4 - Prob. 9ATS
Ch. 1.5 - Prob. 5LTSCh. 1.5 - Prob. 10PTSCh. 1.5 - Prob. 11ATSCh. 1.5 - Prob. 12ATSCh. 1.6 - Prob. 6LTSCh. 1.6 - Prob. 14ATSCh. 1.7 - Prob. 7LTSCh. 1.7 - Prob. 17ATSCh. 1.10 - Prob. 18CCCh. 1.10 - Prob. 20CCCh. 1.10 - Prob. 8LTSCh. 1.10 - Prob. 21PTSCh. 1.10 - Nemotin is a compound that was first isolated from...Ch. 1.10 - Prob. 23CCCh. 1.11 - Prob. 9LTSCh. 1.11 - Prob. 24PTSCh. 1.11 - Prob. 25PTSCh. 1.11 - Prob. 26PTSCh. 1.11 - Prob. 27ATSCh. 1.12 - Prob. 10LTSCh. 1.12 - Prob. 29ATSCh. 1.13 - Prob. 11LTSCh. 1.13 - Prob. 31ATSCh. 1 - Prob. 32PPCh. 1 - Prob. 33PPCh. 1 - Prob. 34PPCh. 1 - Prob. 35PPCh. 1 - Prob. 36PPCh. 1 - Prob. 37PPCh. 1 - Prob. 38PPCh. 1 - Prob. 39PPCh. 1 - Prob. 40PPCh. 1 - Prob. 41PPCh. 1 - Prob. 42PPCh. 1 - Prob. 44PPCh. 1 - Prob. 45PPCh. 1 - Prob. 46PPCh. 1 - Prob. 47PPCh. 1 - Prob. 48PPCh. 1 - Prob. 49PPCh. 1 - Prob. 50PPCh. 1 - Prob. 51PPCh. 1 - Prob. 52PPCh. 1 - Prob. 53PPCh. 1 - Prob. 54PPCh. 1 - Nicotine is an addictive substance found in...Ch. 1 - Prob. 56PPCh. 1 - Prob. 57PPCh. 1 - Prob. 59PPCh. 1 - Prob. 63ASPCh. 1 - Prob. 64ASPCh. 1 - Prob. 66ASPCh. 1 - Prob. 69ASPCh. 1 - Prob. 71ASPCh. 1 - Prob. 72ASPCh. 1 - Prob. 75IP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a. OH H₂N-O -Ph H+ acyclic productarrow_forwardeks.com/aleksogi/x/sl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvTCeeBZbufuBYTI0Hz7m7D3ZS17Hd6m-HIl6n52njJN-TXdQA2X9yID-1SWQJTgnjARg30 111 States of Matter Understanding conceptual components of the enthalpy of solution 0/5 Ge A small amount of acetonitrile (CH, CN) is dissolved in a large amount of water. Imagine separating this process into the four stages sketched below. (These sketches show only a portion of the substances, so you can see the density and distribution of atoms and molecules in them.) CH,CN H₂O B 88 C Use these sketches to answer the questions in the table below. The enthalpy of solution AH is negative soln when CH3CN dissolves in water. Use this information to list the stages in order of increasing enthalpy. Would heat be absorbed or released if the system moved from Stage C to D? What force would oppose or favor the system moving from Stage C to D? Check all that apply. 1 absorbed O released neither absorbed nor released. none O ionic bonding force covalent bonding force…arrow_forwardIn a system with an anodic overpotential, the variation of ŋ as a function of the current density: 1. at low fields is linear 2. at higher fields, it follows Tafel's law Find the range of current densities for which the overpotential has the same value as when calculated for cases 1 and 2 (maximum relative difference of 5% with respect to the behavior for higher fields). To which overpotential range does this correspond? Data: 10 = 1.5 mA cm², T = 300°C, ẞ = 0.64, R = 8.314 J K 1 mol¹ and F = 96485 C mol-1.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY