
Without referring to your textbook or a periodic table, write the full electron configuration, the orbital box diagram, and the noble gas shorthand configuration for the elements with the following
msp;
(a)
Interpretation:
The electronic configuration of the given element, the orbital box diagram and the noble gas shorthand configuration for the elements are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electronic configuration. The description of every electron in an orbital is given by the electronic configuration of that atom.
Answer to Problem 97AP
The electronic configuration of the given element with
Explanation of Solution
The electronic configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is shown in figure 1.
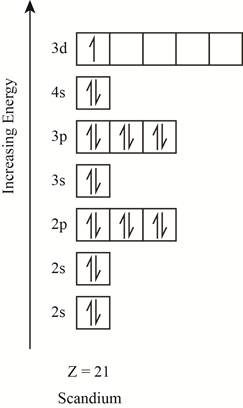
Figure 1
The electronic configuration of the given element can also be written as
(b)
Interpretation:
The electronic configuration of the given element, the orbital box diagram and the noble gas shorthand configuration for the elements are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electronic configuration. The description of every electron in an orbital is given by the electronic configuration of that atom.
Answer to Problem 97AP
The electronic configuration of the given element with
Explanation of Solution
The electronic configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is shown in figure 2.
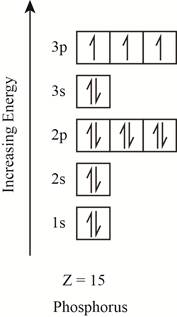
Figure 2
The electronic configuration of the given element can also be written as
(c)
Interpretation:
The electronic configuration of the given element, the orbital box diagram and the noble gas shorthand configuration for the elements are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electronic configuration. The description of every electron in an orbital is given by the electronic configuration of that atom.
Answer to Problem 97AP
The electronic configuration of the given element with
Explanation of Solution
The electronic configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is shown in figure 3.
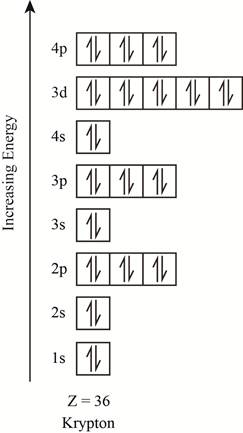
Figure 3
The electronic configuration of the given element can also be written as
(d)
Interpretation:
The electronic configuration of the given element, the orbital box diagram and the noble gas shorthand configuration for the elements are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electronic configuration. The description of every electron in an orbital is given by the electronic configuration of that atom.
Answer to Problem 97AP
The electronic configuration of the given element with
Explanation of Solution
The electronic configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is shown in figure 4.
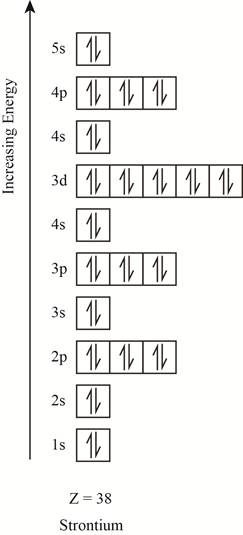
Figure 4
The electronic configuration of the given element can also be written as
(e)
Interpretation:
The electronic configuration of the given element, the orbital box diagram and the noble gas shorthand configuration for the elements are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electronic configuration. The description of every electron in an orbital is given by the electronic configuration of that atom.
Answer to Problem 97AP
The electronic configuration of the given element with
Explanation of Solution
The electronic configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is shown in figure 5.
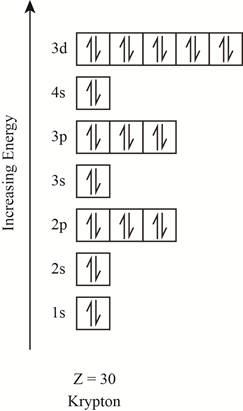
Figure 5
The electronic configuration of the given element can also be written as
Want to see more full solutions like this?
Chapter 11 Solutions
Introductory Chemistry: A Foundation
- Treatment of 2-phenylpropan-2-amine with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. ? NH2 Br Br Propose a structural formula for compound A. You do not have to explicitly draw H atoms. You do not have to consider stereochemistry. In cases where there is more than one answer, just draw one. R3N C14H19NO2 + 2 R3NH*Br Aarrow_forwardCorrectly name this compound using the IUPAC naming system by sorting the components into the correct order. Br IN Ν Harrow_forwardHow is the radical intermediate for this structure formed? Can you please draw arrows from the first radical to the resonance form that would result in this product? I'm lost.arrow_forward
- Part VI. (a) calculate the λ max of the compound using woodward - Fieser rules. (b) what types of electronic transitions are present in the compound? (c) what are the prominent peaks in the IR spectrum of the compound?arrow_forwardDon't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- ↑ 0 Quiz List - RCC430M_RU05 X Aktiv Learning App × Qdraw resonance structure ×Q draw resonance structure xb My Questions | bartleby ×+ https://app.aktiv.com Draw a resonance structure of pyrrole that has the same number of pi bonds as the original structure. Include all lone pairs in your structure. + N H a 5 19°F Cloudy Q Search Problem 12 of 15 Atoms, Bonds and Rings Charges and Lone Pairs myhp हजु Undo Reset Remove Done Submit Drag To Pan 2:15 PM 1/25/2025arrow_forwardDon't used hand raitingarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





