
Study Guide for Chemistry: The Central Science
14th Edition
ISBN: 9780134554075
Author: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus, James C. Hill
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 78AE
The table below shows the normal boiling points of benzene and benzene derivatives.
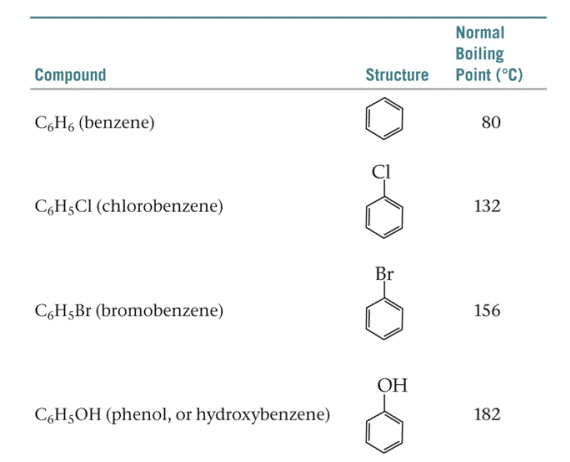
- How many of these compounds exhibit dispersion interaction?
- How many of these compounds exhibit dipole-dipole interaction?
- How many of these compounds exhibit hydrogen bonding?
- Why is the boiling point of bromobenzene higher than that of chlorobenzene?
- Why is the boiling point of phenol the highest of all?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The boiling points of three compounds are tabulated here.
Molar Mass
Boiling Point
2-hexanone
100.16
128°C
heptane
100.20
98°C
1-hexanol
102.17
156°C
Answer the following questions without looking up the
structures for these molecules: Which compound experiences
hydrogen bonding? Which compound is polar but does not
experience hydrogen bonding? Which is neither polar nor
capable of hydrogen bonding? Explain your answers.
1. Each of the following substances is a liquid at -50°C. Place these liquids in order of increasing vapor pressure.
dimethyl ether (CH3OCH3), propane (C3H8), ethanol (CH3CH₂OH)
Essential oils are concentrated liquid containing metabolites extracted from plant sources and are widely used in the production of perfumes. The structures of some of these compounds present in essential oils are presented below.
Arrange the given compounds in terms of increasing boiling points.
An equal volume of COMPOUNDS A, B and C were stored in separate beakers which were left uncovered at room temperature.
If the three liquids were allowed to stand for about 10 minutes, which of the following will have the least amount of liquid remaining in the container?
What physical property is the basis of the answer?
Among the three liquids, what is the most recommended ingredient of a perfume that will have the most lasting scent.
Chapter 11 Solutions
Study Guide for Chemistry: The Central Science
Ch. 11.2 - Which of the following substances is most likely...Ch. 11.2 - Prob. 11.1.2PECh. 11.3 - Prob. 11.2.1PECh. 11.3 - Prob. 11.2.2PECh. 11.4 - What information about water is needed to...Ch. 11.4 - Prob. 11.3.2PECh. 11.5 -
In the mountains, water in an open container will...Ch. 11.5 - Prob. 11.4.2PECh. 11.6 - Prob. 11.5.1PECh. 11.6 - Prob. 11.5.2PE
Ch. 11.7 - Liquid crystalline phases are produced by which of...Ch. 11.7 - Prob. 11.6.2PECh. 11 - Prob. 1DECh. 11 - Prob. 1ECh. 11 - Prob. 2ECh. 11 - Prob. 3ECh. 11 - If 42.0 kj of heat is added to a 32.0-g sample of...Ch. 11 - Prob. 5ECh. 11 - The molecules have the same molecular formula...Ch. 11 - Prob. 7ECh. 11 - Prob. 8ECh. 11 - Prob. 9ECh. 11 - Prob. 10ECh. 11 - Prob. 11ECh. 11 - Prob. 12ECh. 11 - Prob. 13ECh. 11 - Prob. 14ECh. 11 - Prob. 15ECh. 11 - Prob. 16ECh. 11 - Describe the intermolecular forces that must be...Ch. 11 - Prob. 18ECh. 11 - List the following molecules in order of...Ch. 11 - True or false: a. For molecules with similar...Ch. 11 - Prob. 21ECh. 11 - Prob. 22ECh. 11 - Prob. 23ECh. 11 - Prob. 24ECh. 11 - Prob. 25ECh. 11 - Prob. 26ECh. 11 - Prob. 27ECh. 11 - Prob. 28ECh. 11 - Prob. 29ECh. 11 - Prob. 30ECh. 11 - A number of salts containing the tetrahedral...Ch. 11 - Prob. 32ECh. 11 - a. What is the relationship between surface...Ch. 11 - Prob. 34ECh. 11 - Prob. 35ECh. 11 - Prob. 36ECh. 11 - The boiling points, surface tension, and...Ch. 11 - Prob. 38ECh. 11 - Prob. 39ECh. 11 - Prob. 40ECh. 11 - Prob. 41ECh. 11 - Prob. 42ECh. 11 - 11.43 For many years drinking water has been...Ch. 11 - Prob. 44ECh. 11 - Prob. 45ECh. 11 - The fluorocarbon compound C2 Cl 3£'y has a normal...Ch. 11 - 11.47 Indicate whether each statement is true or...Ch. 11 - Prob. 48ECh. 11 - 11,49 Which of the following affects the vapor...Ch. 11 - Prob. 50ECh. 11 - Prob. 51ECh. 11 - Prob. 52ECh. 11 - Prob. 53ECh. 11 - Prob. 54ECh. 11 - Prob. 55ECh. 11 - Prob. 56ECh. 11 - Prob. 57ECh. 11 - Prob. 58ECh. 11 - Prob. 59ECh. 11 - Prob. 60ECh. 11 - Prob. 61ECh. 11 - Prob. 62ECh. 11 - Prob. 63ECh. 11 - Prob. 64ECh. 11 - In terms of the arrangement and freedom of motion...Ch. 11 - Prob. 66ECh. 11 - Prob. 67ECh. 11 - Prob. 68ECh. 11 - Prob. 69ECh. 11 - Prob. 70ECh. 11 - Prob. 71ECh. 11 - Prob. 72ECh. 11 - Prob. 73AECh. 11 - Prob. 74AECh. 11 - Prob. 75AECh. 11 - Prob. 76AECh. 11 - Prob. 77AECh. 11 - The table below shows the normal boiling points of...Ch. 11 - Prob. 79AECh. 11 - Prob. 80AECh. 11 - Prob. 81AECh. 11 - Prob. 82AECh. 11 - Prob. 83AECh. 11 - Prob. 84AECh. 11 - Prob. 85AECh. 11 - Prob. 86AECh. 11 - Prob. 87AECh. 11 - Prob. 88AECh. 11 - Prob. 89AECh. 11 - Prob. 90IECh. 11 - Prob. 91IECh. 11 - Prob. 92IECh. 11 - 11.93 The vapor pressure of ethanol (C2H5OH) at 19...Ch. 11 - Prob. 94IECh. 11 - Prob. 95IECh. 11 - Prob. 96IECh. 11 - Prob. 97IE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate whether each of the following statements concerning boiling and boiling point is true or false. a. A liquid can be made to boil at temperatures higher than its normal boiling point. b. A liquid can be made to boil at temperatures lower than its normal boiling point. c. In a boiling liquid, vapor formation occurs within the body of the liquid. d. To compare the boiling points of two different liquids, the external pressure should be the same.arrow_forwardWhat are intermolecular forces? How do they differ from intramolecular forces? What are dipole-dipole forces? How do typical dipole-dipole forces differ from hydrogen bonding interactions? In what ways are they similar? What are London dispersion forces? How do typical London dispersion forces differ from dipole-dipole forces? In what ways are they similar? Describe the relationship between molecular size and strength of London dispersion forces. Place the major types of intermolecular forces in order of increasing strength. Is there some overlap? That is, can the strongest London dispersion forces be greater than some dipole-dipole forces? Give an example of such an instance.arrow_forwardWhich contains the compounds listed correctly in order of increasing boiling points? (a) N2CS2H2OKCl (b) H2ON2CS2KCl (c) N2KClCS2H2O (d) CS2N2KClH2O (e) KClH2OCS2N2arrow_forward
- True or false? Methane (CH4) is more likely In form stronger hydrogen bonding than is water because each methane molecule has twice as many hydrogen alums. Provide a concise explanation of hydrogen bonding to go with your answer.arrow_forwardHomologous series The alkanes are a homologous series of compounds containing only carbon and hydrogen that have the general formula C,H2n+2. Members of this series include butane (C,H10), 3,3-dimethylpentane (C,H16), hexane (C H14), and heptane ( CH16). Part A The boiling points for a set of compounds in a homologous series can be qualitatively predicted using intermolecular force strengths. Using their condensed structural formulas, rank the homologous series for a set of alkanes by their boiling point Rank from highest to lowest boiling point. To rank items as equivalent, overlap them.arrow_forwardEssential oils are concentrated liquid containing metabolites extracted from plant sources and are widely used in the production of perfumes. The structures of some of these compounds present in essential oils are presented below. Arrange the given compounds in terms of increasing boiling points.arrow_forward
- This graph shows how the vapor pressure of three liquids varies with temperature: vapor pressure, torr 900- 800 700 600- 500 400- 3001 200 1002 0. 100 110 120 Which liquid is the most volatile? Which is the least volatile? 130 temperature, °C Use the graph to answer the following questions: What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be -ethylbenzene -acetic acid pyrrole Suppose a beaker of acetic acid is put inside a sealed tank containing acetic acid gas at 104. degree C and 478. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? 140 most volatile: least volatile: ethylbenzene: acetic acid: pyrrole: O more O less O the same choose one choose one °C 0 °℃ °℃ X ✪ ✪arrow_forwardRank compounds in order of decreasing of their normal boiling points. H2O, Al2O3, Br2, F2, NaCl, IClarrow_forwardThis graph shows how the vapor pressure of three liquids varies with temperature: 900 800 700- 600 500 400 300 - cyclohexane 200 methanol 100 - isopropyl acetate 50 60 80 90 temperature, °C Use the graph to answer the following questions: Which liquid is the most volatile? most volatile: choose one Which is the least volatile? least volatile: choose one cyclohexane: What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be graded correct. methanol: isopropyl acetate: O more Suppose a beaker of methanol is put inside a sealed tank containing methanol gas at 53. degree C and 222. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? less the same vapor pressure, torrarrow_forward
- Which one of the following exhibits dipole-dipole attraction between molecules? BeH2 C7H16 SbF5 N2 HFarrow_forwardThis graph shows how the vapor pressure of three liquids varies with temperature: 900 800 700 600 500 400 300 - cyclohexanone benzaldehyde dimethyl sulfoxide 200 100 150 160 170 180 190 temperature, °C Use the graph to answer the following questions: Which liquid is the most volatile? most volatile: choose one Which is the least volatile? least volatile: choose one cyclohexanone: What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be graded correct. benzaldehyde: dimethyl sulfoxide: | °C Suppose a beaker of benzaldehyde is put inside a sealed tank containing benzaldehyde gas at 169. degree C and 704. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? more less the same vapor pressure, torrarrow_forwardwhich of the following should have the lowest boiling point? NH3 N2 Na2S H2O HFarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Liquids: Crash Course Chemistry #26; Author: Crash Course;https://www.youtube.com/watch?v=BqQJPCdmIp8;License: Standard YouTube License, CC-BY
Chemistry of Group 16 elements; Author: Ch-11 Chemical Engg, Chemistry and others;https://www.youtube.com/watch?v=5B1F0aDgL6s;License: Standard YouTube License, CC-BY