
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
3rd Edition
ISBN: 2818440059223
Author: Hewitt
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 55TE
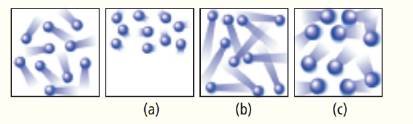
The left most diagram below shows the moving particles of a gas within a rigid container. Which of the three boxes on the right-(a), (b), or (c)-best represents this material upon the addition of heat?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
No chatgpt pls will upvote
A cylinder with a piston contains 0.153 mol of
nitrogen at a pressure of 1.83×105 Pa and a
temperature of 290 K. The nitrogen may be
treated as an ideal gas. The gas is first compressed
isobarically to half its original volume. It then
expands adiabatically back to its original volume,
and finally it is heated isochorically to its original
pressure.
Part A
Compute the temperature at the beginning of the adiabatic expansion.
Express your answer in kelvins.
ΕΠΙ ΑΣΦ
T₁ =
?
K
Submit
Request Answer
Part B
Compute the temperature at the end of the adiabatic expansion.
Express your answer in kelvins.
Π ΑΣΦ
T₂ =
Submit
Request Answer
Part C
Compute the minimum pressure.
Express your answer in pascals.
ΕΠΙ ΑΣΦ
P =
Submit
Request Answer
?
?
K
Pa
Learning Goal:
To understand the meaning and the basic applications of
pV diagrams for an ideal gas.
As you know, the parameters of an ideal gas are
described by the equation
pV = nRT,
where p is the pressure of the gas, V is the volume of
the gas, n is the number of moles, R is the universal gas
constant, and T is the absolute temperature of the gas. It
follows that, for a portion of an ideal gas,
pV
= constant.
Τ
One can see that, if the amount of gas remains constant,
it is impossible to change just one parameter of the gas:
At least one more parameter would also change. For
instance, if the pressure of the gas is changed, we can
be sure that either the volume or the temperature of the
gas (or, maybe, both!) would also change.
To explore these changes, it is often convenient to draw a
graph showing one parameter as a function of the other.
Although there are many choices of axes, the most
common one is a plot of pressure as a function of
volume: a pV diagram.
In this problem, you…
Chapter 11 Solutions
CONCEPTUAL INTEGRATED SCIENCE (PEARSON+
Ch. 11 - Prob. 1RCCCh. 11 - Prob. 2RCCCh. 11 - Prob. 3RCCCh. 11 - Prob. 4RCCCh. 11 - Prob. 5RCCCh. 11 - How are the particles in a solid arranged...Ch. 11 - Which occupies the greatest volume: 1 gram of ice,...Ch. 11 - What is it called when evaporation takes place...Ch. 11 - How is sublimation different from evaporation?Ch. 11 - Prob. 10RCC
Ch. 11 - How much heat is needed to melt 1 gram of ice?...Ch. 11 - What happens to the chemical identity of a...Ch. 11 - What is a physical property? A chemical property?Ch. 11 - What is a chemical bond?Ch. 11 - What changes during a chemical reaction?Ch. 11 - Why is the freezing of water considered to be a...Ch. 11 - Prob. 17RCCCh. 11 - Why is the rusting of iron considered to be a...Ch. 11 - Prob. 19RCCCh. 11 - What is the difference between an element and a...Ch. 11 - How many atoms are in one molecule of H3PO4?Ch. 11 - How many atoms of each element are in one molecule...Ch. 11 - What does the chemical formula of a substance tell...Ch. 11 - Prob. 24RCCCh. 11 - Prob. 25RCCCh. 11 - Prob. 26RCCCh. 11 - What is the chemical formula for the compound...Ch. 11 - Why are common names often used for chemical...Ch. 11 - How soon will nanotechnology give rise to...Ch. 11 - Prob. 30TISCh. 11 - Who is the ultimate expert at nanotechnology?Ch. 11 - Prob. 38TCCh. 11 - Rank these substances in order of increasing...Ch. 11 - Rank the following physical and chemical changes...Ch. 11 - Rank these compounds in order of increasing number...Ch. 11 - How has chemistry influenced our modern...Ch. 11 - While visiting a foreign country, a foreign...Ch. 11 - If someone is able to explain an idea to you using...Ch. 11 - What is the best way to really prove to yourself...Ch. 11 - Prob. 46TECh. 11 - Prob. 47TECh. 11 - What is found between two adjacent molecules of a...Ch. 11 - You combine 50mL of water with 50mL of purified...Ch. 11 - Prob. 50TECh. 11 - Which has stronger attractions among its...Ch. 11 - Prob. 52TECh. 11 - Is it possible for air to be in liquid phase?...Ch. 11 - Prob. 54TECh. 11 - The left most diagram below shows the moving...Ch. 11 - The leftmost diagram here shows two phases of a...Ch. 11 - A cotton ball is dipped in alcohol and wiped...Ch. 11 - A skillet is lined with a thin layer of cooking...Ch. 11 - A cotton ball is dipped in alcohol is wiped across...Ch. 11 - Use exercise 58 as an analogy to describe what...Ch. 11 - Prob. 61TECh. 11 - Prob. 62TECh. 11 - Prob. 63TECh. 11 - Why are physical changes typically easier to...Ch. 11 - Prob. 65TECh. 11 - Prob. 66TECh. 11 - Each night you measure your height just before...Ch. 11 - State whether each of the following is an example...Ch. 11 - State whether each of the following is an example...Ch. 11 - How is sugar dissolving in water an example of a...Ch. 11 - Why is the air over a campfire always moist?Ch. 11 - Prob. 72TECh. 11 - Prob. 73TECh. 11 - Each sphere in the diagrams shown here represents...Ch. 11 - Is aging primarily an example of a physical or a...Ch. 11 - Is nuclear fusion, as described in Chapter 10, an...Ch. 11 - Prob. 77TECh. 11 - Prob. 78TECh. 11 - Oxygen atoms are used to make water molecules....Ch. 11 - Oxygen, O2, is certainly good for you. Does it...Ch. 11 - Prob. 81TECh. 11 - Prob. 82TECh. 11 - Which of the following boxes contains only an...Ch. 11 - Prob. 84TECh. 11 - Prob. 85TECh. 11 - What is the chemical name for a compound with the...Ch. 11 - Prob. 87TECh. 11 - Prob. 88TECh. 11 - Is nanotechnology the result of basic or applied...Ch. 11 - How does a scanning probe microscope differ from...Ch. 11 - People often behave differently in a group...Ch. 11 - Prob. 92TECh. 11 - Medicines, such as pain relievers and...Ch. 11 - Your friend smells cinnamon coming from an...Ch. 11 - The British diplomat, physicist, and...Ch. 11 - Prob. 96TDICh. 11 - A calculator is useful but certainly not exciting....Ch. 11 - How might speculations about potential dangers of...Ch. 11 - Over the past 20 years, the average life...Ch. 11 - Prob. 100TDICh. 11 - Prob. 1RATCh. 11 - The molecules in a small collection of molecules...Ch. 11 - The phase in which atoms and molecules no longer...Ch. 11 - Prob. 4RATCh. 11 - Prob. 5RATCh. 11 - Prob. 6RATCh. 11 - Which is an example of a chemical change? a Water...Ch. 11 - If you burn 50kg of wood and produce 10g of ash,...Ch. 11 - If you have one molecule of TiO2, how many...Ch. 11 - Prob. 10RAT
Additional Science Textbook Solutions
Find more solutions based on key concepts
Endospore formation is called (a) _____. It is initiated by (b) _____. Formation of a new cell from an endospor...
Microbiology: An Introduction
1.3 Obtain a bottle of multivitamins and read the list of ingredients. What are four chemicals from the list?
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
53. This reaction was monitored as a function of time:
A plot of In[A] versus time yields a straight ...
Chemistry: Structure and Properties (2nd Edition)
All of the following terms can appropriately describe humans except: a. primary consumer b. autotroph c. hetero...
Human Biology: Concepts and Current Issues (8th Edition)
Q2. Which statement best defines chemistry?
a. The science that studies solvents, drugs, and insecticides
b. Th...
Introductory Chemistry (6th Edition)
The bioremediation process shown in the photograph is used to remove benzene and other hydrocarbons from soil c...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Learning Goal: To understand the meaning and the basic applications of pV diagrams for an ideal gas. As you know, the parameters of an ideal gas are described by the equation pV = nRT, where p is the pressure of the gas, V is the volume of the gas, n is the number of moles, R is the universal gas constant, and T is the absolute temperature of the gas. It follows that, for a portion of an ideal gas, pV = constant. T One can see that, if the amount of gas remains constant, it is impossible to change just one parameter of the gas: At least one more parameter would also change. For instance, if the pressure of the gas is changed, we can be sure that either the volume or the temperature of the gas (or, maybe, both!) would also change. To explore these changes, it is often convenient to draw a graph showing one parameter as a function of the other. Although there are many choices of axes, the most common one is a plot of pressure as a function of volume: a pV diagram. In this problem, you…arrow_forward■ Review | Constants A cylinder with a movable piston contains 3.75 mol of N2 gas (assumed to behave like an ideal gas). Part A The N2 is heated at constant volume until 1553 J of heat have been added. Calculate the change in temperature. ΜΕ ΑΣΦ AT = Submit Request Answer Part B ? K Suppose the same amount of heat is added to the N2, but this time the gas is allowed to expand while remaining at constant pressure. Calculate the temperature change. AT = Π ΑΣΦ Submit Request Answer Provide Feedback ? K Nextarrow_forward4. I've assembled the following assortment of point charges (-4 μC, +6 μC, and +3 μC) into a rectangle, bringing them together from an initial situation where they were all an infinite distance away from each other. Find the electric potential at point "A" (marked by the X) and tell me how much work it would require to bring a +10.0 μC charge to point A if it started an infinite distance away (assume that the other three charges remains fixed). 300 mm -4 UC "A" 0.400 mm +6 UC +3 UC 5. It's Friday night, and you've got big party plans. What will you do? Why, make a capacitor, of course! You use aluminum foil as the plates, and since a standard roll of aluminum foil is 30.5 cm wide you make the plates of your capacitor each 30.5 cm by 30.5 cm. You separate the plates with regular paper, which has a thickness of 0.125 mm and a dielectric constant of 3.7. What is the capacitance of your capacitor? If you connect it to a 12 V battery, how much charge is stored on either plate? =arrow_forward
- Learning Goal: To understand the meaning and the basic applications of pV diagrams for an ideal gas. As you know, the parameters of an ideal gas are described by the equation pV = nRT, where p is the pressure of the gas, V is the volume of the gas, n is the number of moles, R is the universal gas constant, and T is the absolute temperature of the gas. It follows that, for a portion of an ideal gas, PV T = constant. One can see that, if the amount of gas remains constant, it is impossible to change just one parameter of the gas: At least one more parameter would also change. For instance, if the pressure of the gas is changed, we can be sure that either the volume or the temperature of the gas (or, maybe, both!) would also change. To explore these changes, it is often convenient to draw a graph showing one parameter as a function of the other. Although there are many choices of axes, the most common one is a plot of pressure as a function of volume: a pV diagram. In this problem, you…arrow_forwardA-e pleasearrow_forwardTwo moles of carbon monoxide (CO) start at a pressure of 1.4 atm and a volume of 35 liters. The gas is then compressed adiabatically to 1/3 this volume. Assume that the gas may be treated as ideal. Part A What is the change in the internal energy of the gas? Express your answer using two significant figures. ΕΠΙ ΑΣΦ AU = Submit Request Answer Part B Does the internal energy increase or decrease? internal energy increases internal energy decreases Submit Request Answer Part C ? J Does the temperature of the gas increase or decrease during this process? temperature of the gas increases temperature of the gas decreases Submit Request Answerarrow_forward
- Your answer is partially correct. Two small objects, A and B, are fixed in place and separated by 2.98 cm in a vacuum. Object A has a charge of +0.776 μC, and object B has a charge of -0.776 μC. How many electrons must be removed from A and put onto B to make the electrostatic force that acts on each object an attractive force whose magnitude is 12.4 N? e (mea is the es a co le E o ussian Number Tevtheel ed Media ! Units No units → answe Tr2Earrow_forward4 Problem 4) A particle is being pushed up a smooth slot by a rod. At the instant when 0 = rad, the angular speed of the arm is ė = 1 rad/sec, and the angular acceleration is = 2 rad/sec². What is the net force acting on the 1 kg particle at this instant? Express your answer as a vector in cylindrical coordinates. Hint: You can express the radial coordinate as a function of the angle by observing a right triangle. (20 pts) Ꮎ 2 m Figure 3: Particle pushed by rod along vertical path.arrow_forward4 Problem 4) A particle is being pushed up a smooth slot by a rod. At the instant when 0 = rad, the angular speed of the arm is ė = 1 rad/sec, and the angular acceleration is = 2 rad/sec². What is the net force acting on the 1 kg particle at this instant? Express your answer as a vector in cylindrical coordinates. Hint: You can express the radial coordinate as a function of the angle by observing a right triangle. (20 pts) Ꮎ 2 m Figure 3: Particle pushed by rod along vertical path.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY