
In a laboratory experiment, an electron with a kinetic energy of 50.5 keV is shot toward another electron initially at rest (Fig. P11.50). (1 eV = 1.602 × 10−19 J) The collision is elastic. The initially moving electron is deflected by the collision.
- a. Is it possible for the initially stationary electron to remain at rest after the collision? Explain.
- b. The initially moving electron is detected at an angle of 40.0° from its original path. What is the speed of each electron after the collision?
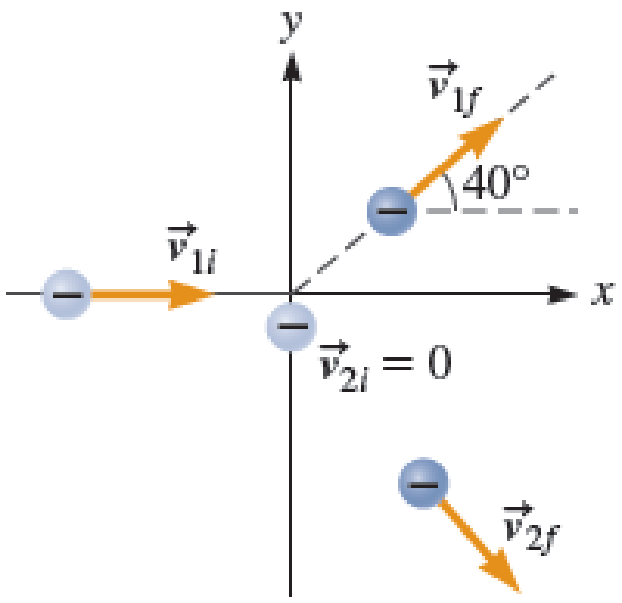
FIGURE P11.50
(a)
Whether it is possible for the initially stationary electron to remain at rest after the collision.
Answer to Problem 50PQ
It is not possible for the initially stationary electron to remain at rest after the collision.
Explanation of Solution
The elastic collision is characterized by the conservation of momentum as well as kinetic energy. For an elastic collision initial momentum of the system before collision should be equal to the momentum of the system after collision.
In the given situation, the initial momentum of the system in
Conclusion:
Thus, it is not possible for the initially stationary electron to remain at rest after the collision.
(b)
The speed of each electron after the collision.
Answer to Problem 50PQ
The speed of initially moving electron after the collision is
Explanation of Solution
The given situation is a two-dimensional elastic collision between two particles of equal mass with initial velocity for one of the particle equal to zero. For such a collision, the angle between the final velocities of the particles after the collision will be equal to
Here,
Rearrange the above equation for
It is given that the value of
Substitute
Write the expression for the conservation of momentum in
Here,
Write the expression for
Here,
Substitute
Write the expression for
Here,
Substitute
Put equations (II) and (III) in equation (I).
The momentum in
Write the expression for the conservation of momentum in
Here,
Write the expression for
Substitute
Write the expression for
The negative sign is due to the fact that the electron 2 moves in negative
Substitute
Put equations (VI) and (VII) in equation (V) and rearrange it for
Put the above equation in equation (IV) and rearrange it for
Put equation (IX) in equation (VIII).
Write the equation for the initial kinetic energy of electron 1.
Here,
Rewrite the above equation for
Conclusion:
It is given that the initial kinetic energy of electron 1 is
Substitute
Substitute
Substitute
Therefore, the speed of initially moving electron after the collision is
Want to see more full solutions like this?
Chapter 11 Solutions
EBK WEBASSIGN FOR KATZ'S PHYSICS FOR SC
- I need help with part B. I cant seem to get the correct answer. Please walk me through what youre doing to get to the answer and what that could bearrow_forwardQuestion 6: Chlorine is widely used to purify municipal water supplies and to treat swimming pool waters. Suppose that the volume of a particular sample of Cl₂ gas is 8.70 L at 895 torr and 24°C. (a) How many grams of Cl₂ are in the sample? ⚫ Atomic mass of CI = 35.453 g/mol • Molar mass of Cl₂ = 2 x 35.453 = 70.906 g/mol Solution: Use the Ideal Gas Law: Step 1: Convert Given Values • Pressure: P = 895 torr → atm PV= = nRT 1 P = 895 × = 1.1789 atm 760 • Temperature: Convert to Kelvin: T24273.15 = 297.15 K • Gas constant: R = 0.0821 L atm/mol. K Volume: V = 8.70 L Step 2: Solve for n . PV n = RT n = (1.1789)(8.70) (0.0821)(297.15) 10.25 n = = 0.420 mol 24.405 Step 3: Calculate Mass of Cl₂ Final Answer: 29.78 g of Cl₂. mass nx M mass= (0.420)(70.906) mass= 29.78 garrow_forwardE1 R₁ w 0.50 20 Ω 12 R₁₂ ww ΒΩ R₂ 60 E3 C RA w 15 Ω E2 0.25 E4 0.75 Ω 0.5 Ωarrow_forward
- What is the force (in N) on the 2.0 μC charge placed at the center of the square shown below? (Express your answer in vector form.) 5.0 με 4.0 με 2.0 με + 1.0 m 1.0 m -40 με 2.0 μCarrow_forwardWhat is the force (in N) on the 5.4 µC charge shown below? (Express your answer in vector form.) −3.1 µC5.4 µC9.2 µC6.4 µCarrow_forwardAn ideal gas in a sealed container starts out at a pressure of 8900 N/m2 and a volume of 5.7 m3. If the gas expands to a volume of 6.3 m3 while the pressure is held constant (still at 8900 N/m2), how much work is done by the gas? Give your answer as the number of Joules.arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning





