
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
7th Edition
ISBN: 9780134240152
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 11, Problem 50P
Interpretation Introduction
Interpretation:
The products of each stages in the given reaction should be identified.
Concept introduction:
Epoxide is highly reactive and it is reaction with hydroxide ion which produces Trans
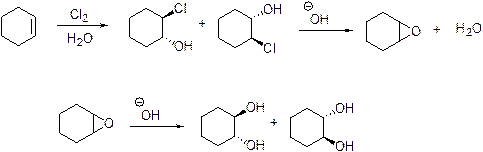
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Steps and explanation please
Steps and explanation please
can you please draw out and list step-by-step the synthetic strategy for this rxn? thank you sm in advance
Chapter 11 Solutions
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
Ch. 11.1 - Why are NH3 and CH3NH2 no longer nucleophiles when...Ch. 11.1 - Prob. 2PCh. 11.1 - Prob. 5PCh. 11.2 - Prob. 7PCh. 11.3 - Prob. 9PCh. 11.3 - Show how 1-propanol can be converted into the...Ch. 11.4 - Which of the following alcohols dehydrates the...Ch. 11.4 - Prob. 12PCh. 11.4 - Prob. 13PCh. 11.4 - Propose a mechanism for each of the following...
Ch. 11.4 - Draw the product of each of the following...Ch. 11.4 - Explain why the following alcohols, when heated...Ch. 11.4 - What stereoisomers are formed in the following...Ch. 11.4 - Prob. 18PCh. 11.4 - What alcohol would you treat with phosphorus...Ch. 11.5 - Prob. 20PCh. 11.6 - Prob. 22PCh. 11.7 - Prob. 24PCh. 11.7 - Would you expect the reactivity of a five-membered...Ch. 11.7 - Prob. 26PCh. 11.7 - What products are obtained from the reaction of...Ch. 11.7 - Prob. 28PCh. 11.7 - Prob. 29PCh. 11.7 - Prob. 30PCh. 11.8 - Prob. 31PCh. 11.8 - Prob. 32PCh. 11.8 - How do the major products obtained from...Ch. 11.8 - Explain why the two arene oxides in Problem 38...Ch. 11.8 - Three arene oxides can be obtained from...Ch. 11.9 - Explain why the half-life (the time it takes for...Ch. 11.10 - Prob. 38PCh. 11.10 - Prob. 39PCh. 11.10 - Prob. 40PCh. 11.10 - Prob. 41PCh. 11.10 - Prob. 42PCh. 11.11 - Using an alkyl halide and a thiol as starting...Ch. 11.11 - The following three nitrogen mustards were studied...Ch. 11.11 - Why is melphalan a good cancer drug?Ch. 11.11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Which compound is more likely to be carcinogenic?Ch. 11 - Prob. 50PCh. 11 - Prob. 51PCh. 11 - Write the appropriate reagent over each arrow.Ch. 11 - What alkenes would you expect to be obtained from...Ch. 11 - Prob. 54PCh. 11 - When heated with H2SO4, both...Ch. 11 - What is the major product obtained from the...Ch. 11 - When deuterated phenanthrene oxide undergoes a...Ch. 11 - An unknown alcohol with a molecular formula of...Ch. 11 - Prob. 59PCh. 11 - Prob. 60PCh. 11 - Propose a mechanism for the following reaction:Ch. 11 - What product would be formed if the four-membered...Ch. 11 - Which of the following ethers would be obtained in...Ch. 11 - Using the given starting material any necessary...Ch. 11 - Prob. 65PCh. 11 - When 3-methyl-2-butanol is heated with...Ch. 11 - Propose a mechanism for each of the following...Ch. 11 - How could you synthesize isopropyl propyl ether,...Ch. 11 - When the following seven-membered ring alcohol is...Ch. 11 - Ethylene oxide reacts readily with HO because of...Ch. 11 - Prob. 71PCh. 11 - Propose a mechanism for each of the following...Ch. 11 - Explain why the acid-catalyzed dehydration of an...Ch. 11 - Triethylene glycol is one of the products obtained...Ch. 11 - Prob. 75PCh. 11 - Prob. 76PCh. 11 - When ethyl ether is heated with excess HI for...Ch. 11 - Propose a mechanism for the following reaction:Ch. 11 - Prob. 79PCh. 11 - An ion with a positively charged nitrogen atom in...Ch. 11 - Propose a mechanism for each of the following...Ch. 11 - Prob. 82PCh. 11 - The following reaction takes place several times...Ch. 11 - A vicinal diol has OH groups on adjacent carbons....Ch. 11 - Prob. 85PCh. 11 - Prob. 86PCh. 11 - Two stereoisomers are obtained from the reaction...Ch. 11 - Propose a mechanism for each or the following...Ch. 11 - Triethylenemelamine (TEM) is an antitumor agent....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Steps and explanations pleasearrow_forwardUse diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forwardDraw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward
- Match the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forwardCan you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward
- 2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forwardconsider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
