
Concept explainers
Give an acceptable IUPAC name for each of the following compounds:
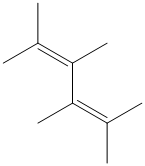
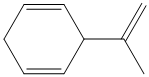
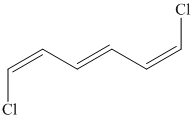
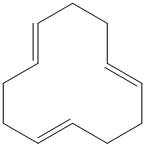
Interpretation:
The acceptable IUPAC name for each of the given compounds is to be deteremined.
Concept introduction:
Alkanes have the general formula
The parent chain in each of hydrocarbon structure is first identified and the chain is numbered such that the substituents attached get lowest numbers.
Prefixes are used to denote the number of identical substituents.
The substituents are written in alphabetical order while writing the IUPAC name.
If the two groups of atoms that are the higher priority are on the same side of the double bond, then the bond is termed as the Z-configuration. While if the two group of higher priority are on the opposite side then the configuration is E-configuration.
Answer to Problem 27P
Solution:
a)
b)
c)
d)
e)
f)
g)
h)
Explanation of Solution
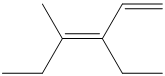
a) The expanded structure for the given structure can be drawn as follows:
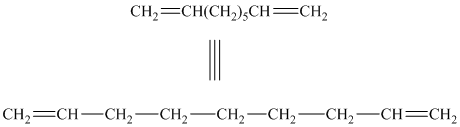
In the above structure, the parent chain is the continuous chain from
Thus, the IUPAC name is
b) The structure for the given compound is as follows:
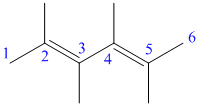
In the above structure, the parent longest chain is from
Thus, the IUPAC name is
c) The structure for the given compound and its expanded structure is as follows:
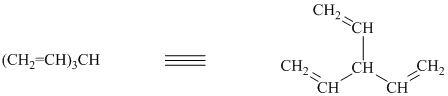
In the above structure, the parent longest chain is from
Thus, the IUPAC name is
d) The structure for the given compound is as follows:
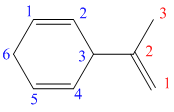
In the above structure, the parent ring contains six carbon atoms, so the parent name of the cycloalkane is cyclohexane. There are two double bonds in the given compound, the first one exist in between
Considering the Cahn–Ingold–Prelog sequence rules, for the cyclohexadiene, the higher priority groups are on the same side in both the double bonds. Hence, both get the stereochemical label as Z.
Thus, the IUPAC name of this structure is
e) The structure for the given compound is as follows:

In the above structure, the parent longest chain is from
For double bonds at
For the internal double bond at carbon
Thus, the IUPAC name is
f) The structure for the given compound is as follows:
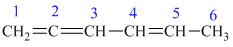
In the above structure, the parent longest chain is from
Thus, the IUPAC name is
g) The structure for the given compound is as follows:
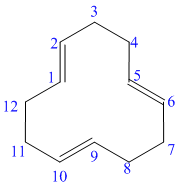
In the above structure, the parent ring contains twelve carbon atoms, so the parent name of the cycloalkane is cyclododecane. There are three double bonds in the given compound, the first one exist in between
For all the three double bonds at
Thus, the IUPAC name of this structure is
h) The structure for the given compound is as follows:
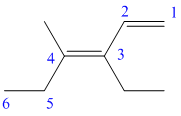
In the above structure, the parent longest chain is from
For the double bonds at
Thus, the IUPAC name is
Want to see more full solutions like this?
Chapter 11 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
- a) A favorable entropy change occurs when ΔS is positive. Does the order of the system increase or decrease when ΔS is positive? (b) A favorable enthalpy change occurs when ΔH is negative. Does the system absorb heat or give off heat when ΔH is negative? (c) Write the relation between ΔG, ΔH, and ΔS. Use the results of parts (a) and (b) to state whether ΔG must be positive or negative for a spontaneous change. For the reaction, ΔG is 59.0 kJ/mol at 298.15 K. Find the value of K for the reaction.arrow_forwardA sample of hydrated magnesium sulfate (MgSO4⋅xH2O) is analyzed using thermogravimetric analysis (TGA). The sample weighs 2.50 g initially and is heated in a controlled atmosphere. As the temperature increases, the water of hydration is released in two stages: (a) The first mass loss of 0.72 g occurs at 150°C, corresponding to the loss of a certain number of water molecules. (b) The second mass loss of 0.90 g occurs at 250°C, corresponding to the loss of the remaining water molecules. The residue is identified as anhydrous magnesium sulfate (MgSO4) Questions: (i) Determine the value of x (the total number of water molecules in MgSO4⋅xH2O) (ii) Calculate the percentage of water in the original sample. Write down the applications of TGA.arrow_forwardThe solubility product of iron(III) hydroxide (Fe(OH)3) is 6.3×10−38. If 50 mL of a 0.001 M FeCl3 solution is mixed with 50 mL of a 0.005 M NaOH solution, will Fe(OH)3 precipitate? Show all step-by-step calculations. To evaluate the equilibrium constant, we must express concentrations of solutes in mol/L, gases in bars, and omit solids, liquids, and solvents. Explain why.arrow_forward
- Predict the major products of this organic reaction.arrow_forward2. Provide the structure of the major organic product in the following reaction. Pay particular attention to the regio- and stereochemistry of your product. H3CO + H CN Aarrow_forwardPredict the major products of the following organic reaction.arrow_forward
- 1) The isoamyl acetate report requires eight paragraphs - four for comparison of isoamyl alcohol and isoamyl acetate (one paragraph each devoted to MS, HNMR, CNMR and IR) and four for comparison of acetic acid and isoamyl acetate ((one paragraph each devoted to MS, HNMR, CNMR and IR. 2) For MS, the differing masses of molecular ions are a popular starting point. Including a unique fragmentation is important, too. 3) For HNMR, CNMR and IR state the peaks that are different and what makes them different (usually the presence or absence of certain groups). See if you can find two differences (in each set of IR, HNMR and CNMR spectra) due to the presence or absence of a functional group. Include peak locations. Alternatively, you can state a shift of a peak due to a change near a given functional group. Including peak locations for shifted peaks, as well as what these peaks are due to. Ideally, your focus should be on not just identifying the differences but explaining them in terms of…arrow_forwardWhat steps might you take to produce the following product from the given starting material? CI Br Он до NH2 NH2arrow_forward1) The isoamyl acetate report requires eight paragraphs - four for comparison of isoamyl alcohol and isoamyl acetate (one paragraph each devoted to MS, HNMR, CNMR and IR) and four for comparison of acetic acid and isoamyl acetate ((one paragraph each devoted to MS, HNMR, CNMR and IR. 2) For MS, the differing masses of molecular ions are a popular starting point. Including a unique fragmentation is important, too. 3) For HNMR, CNMR and IR state the peaks that are different and what makes them different (usually the presence or absence of certain groups). See if you can find two differences (in each set of IR, HNMR and CNMR spectra) due to the presence or absence of a functional group. Include peak locations. Alternatively, you can state a shift of a peak due to a change near a given functional group. Including peak locations for shifted peaks, as well as what these peaks are due to. Ideally, your focus should be on not just identifying the differences but explaining them in terms of…arrow_forward
- №3 Fill in the below boxes. HN 1. LAH 2. H3O+ NH2arrow_forwardFor the photochemical halogenation reaction below, draw both propagation steps and include the mechanism arrows for each step. H CH ot CH3 CI-CI MM hv of CH H-CI CH3 2nd attempt See Periodic Table See Hint Draw only radical electrons; do not add lone pair electrons. Note that arrows cannot meet in "space," and must end at either bonds or at atoms. 1 i Add the missing curved arrow notation to this propagation step. 20 H ن S F P H CI Br 品arrow_forwardThe radical below can be stabilized by resonance. 4th attempt Draw the resulting resonance structure. DOCEarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
