
(a)
Interpretation:
The synthesis of
Concept introduction:
Oxymercuration reaction is a type of reaction in which an
Answer to Problem 11.61AP
The synthesis of
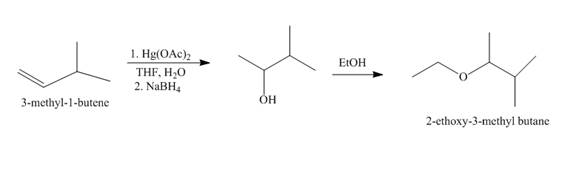
Explanation of Solution
The mercuric acetate

Figure 1
The route of synthesis of the formation of
(b)
Interpretation:
The synthesis of
Concept introduction:
The substances which on addition removes oxygen atom or hydrogen atom from the other substance, that is, reduces the other substances are known as reducing agents. Reducing agents themselves get oxidized. Strong reducing agents like
Answer to Problem 11.61AP
The synthesis of
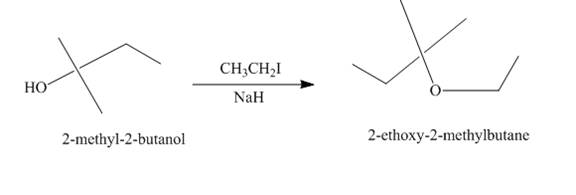
Explanation of Solution
The treatment of
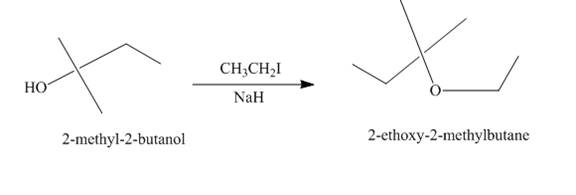
Figure 2
The route of synthesis of the formation of
(c)
Interpretation:
The synthesis of
Concept introduction:
Grignard reagents are
Answer to Problem 11.61AP
The synthesis of
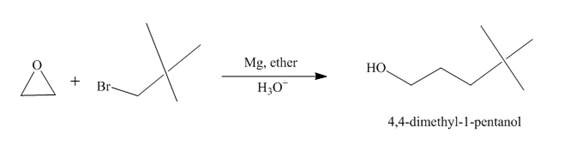
Explanation of Solution
The reaction of tert-butyl bromide with

Figure 3
The route of synthesis of the formation of
(d)
Interpretation:
The synthesis of the given compound from the compounds that contain
Concept introduction:
Oxidation is the process in which there is an addition of oxygen atom or removal of hydrogen atom. The agents used for the oxidation reaction is known as oxidizing agents. It oxidizes the other substance and gets reduced in the reaction. The common oxidizing agents are nitric acid, oxygen and potassium dichromate.
Answer to Problem 11.61AP
The synthesis of the given compound from the compounds that contain
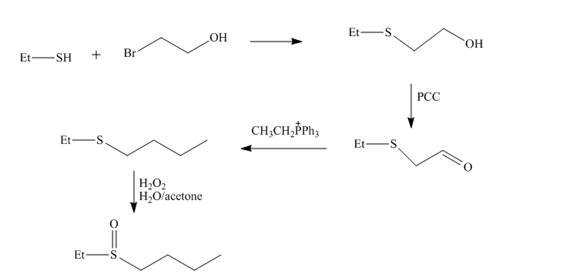
Explanation of Solution
The reaction of ethnethiol with
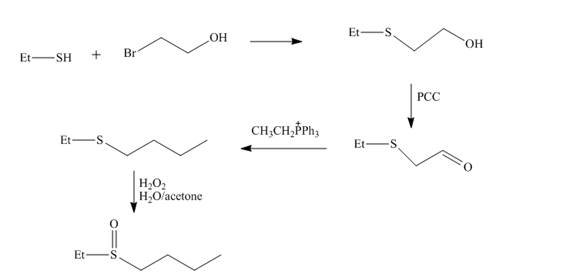
Figure 4
The synthesis of the given compound from the compounds that contain
(e)
Interpretation:
The synthesis of the given compound from an alkene is to be drawn.
Concept introduction:
Oxidation is the process in which there is an addition of oxygen atom or removal of hydrogen atom. The agents used for the oxidation reaction is known as oxidizing agents. It oxidizes the other substance and gets reduced in the reaction. The common oxidizing agents are nitric acid, oxygen and potassium dichromate.
Answer to Problem 11.61AP
The synthesis of the given compound from an alkene is shown below.
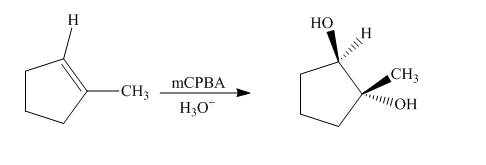
Explanation of Solution
The reaction of

Figure 5
The synthesis of the given compound from an alkene is shown in Figure 5.
(f)
Interpretation:
The synthesis of cyclohexyl isopropyl ether from cyclohexene is to be drawn.
Concept introduction:
Oxidation is the process in which there is an addition of oxygen atom or removal of hydrogen atom. The agents used for the oxidation reaction is known as oxidizing agents. It oxidizes the other substance and gets reduced in the reaction. The common oxidizing agents are nitric acid, oxygen and potassium dichromate.
Answer to Problem 11.61AP
The synthesis of cyclohexyl isopropyl ether from cyclohexene is shown below.
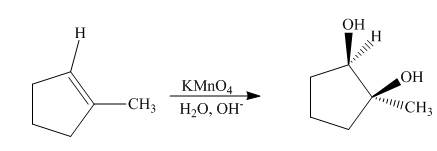
Explanation of Solution
The compound,

Figure 6
The synthesis of cyclohexyl isopropyl ether from cyclohexene is shown Figure 6.
(g)
Interpretation:
The synthesis of cyclohexyl isopropyl ether from cyclohexene is to be drawn.
Concept introduction:
Oxymercuration reaction is a type of reaction in which an alkene gets converted to alcohol. The mercuric acetate is used in the reaction as a reagent. This reagent attacks the alkene to form a cyclic intermediate compound which further undergoes reduction to form alcohol.
Answer to Problem 11.61AP
The synthesis of cyclohexyl isopropyl ether from cyclohexene is shown below.
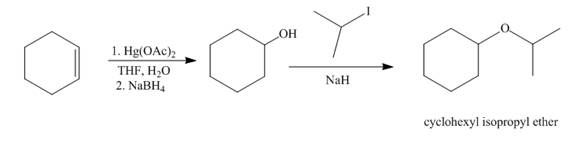
Explanation of Solution
The mercuric acetate

Figure 7
The synthesis route of cyclohexyl isopropyl ether from cyclohexene is shown in Figure 7.
(h)
Interpretation:
The synthesis of the given compound from
Concept introduction:
Grignard reagents are organometallic compounds which are prepared using alkyl halides in the presence of magnesium metal in dry ether. These reagents act as strong nucleophiles and bases
Answer to Problem 11.61AP
The synthesis of the given compound from
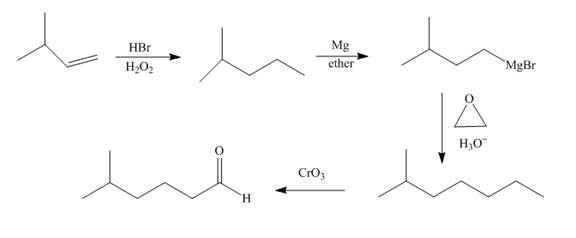
Explanation of Solution
Bromination of alkene occurs with
Grigmard reagent reacts with the ethylene oxide followed by the hydrolysis to form an alcohol which upon oxidation by
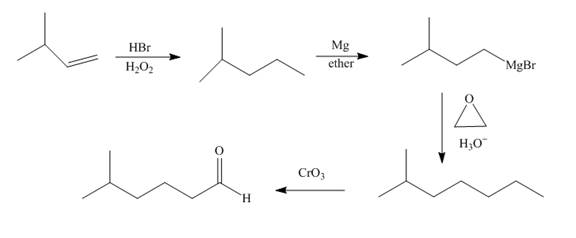
Figure 8
The synthesis of the given compound from
(i)
Interpretation:
The synthesis of the given compound from
Concept introduction:
An
Oxidation is the process in which there is an addition of oxygen atom or removal of hydrogen atom. The agents used for the oxidation reaction is known as oxidizing agents. It oxidizes the other substance and gets reduced in the reaction. The common oxidizing agents are nitric acid, oxygen and potassium dichromate.
Answer to Problem 11.61AP
The synthesis of the given compound from
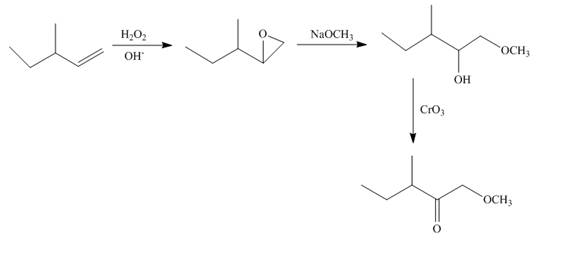
Explanation of Solution
Epoxidation of

Figure 9
The synthesis route of the given compound from
(j)
Interpretation:
The synthesis of the given compound from
Concept introduction:
An epoxide is cyclic ether. It can be prepare by the reaction of alkene with percarboxylic acid with removal of carboxylic acid. An epoxide undergoes ring opening reactions with an acid to give a product with inversion configuration.
Oxidation is the process in which there is an addition of oxygen atom or removal of hydrogen atom. The agents used for the oxidation reaction is known as oxidizing agents. It oxidizes the other substance and get reduced in the reaction. The common oxidizing agents are nitric acid, oxygen and potassium dichromate.
Answer to Problem 11.61AP
The synthesis of the given compound from
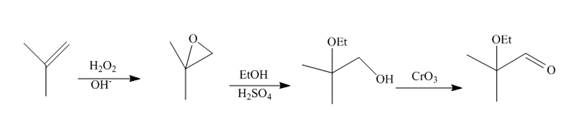
Explanation of Solution
Epoxidation of

Figure 10
The synthesis route of the given compound from
(k)
Interpretation:
The synthesis of given compound from the allyl chloride is to be drawn.
Concept introduction:
The nucleophilic substitution reactions depend upon the nucleophilicity and concentration of the nucleophile. There are two types of nucleophilic substitution reaction.
The
Answer to Problem 11.61AP
The synthesis of given compound from the allyl chloride is shown below.
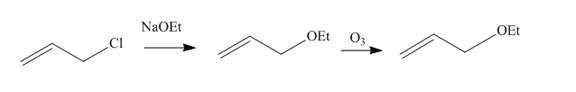
Explanation of Solution
Allyl chloride reacts with

Figure 11
The synthesis of given compound from the allyl chloride is shown in Figure 11.
(l)
Interpretation:
The synthesis of the given compound is to be drawn.
Concept introduction:
Wolff Kishner Reduction is a reaction in which aldehydes and ketones are converted into
Answer to Problem 11.61AP
The synthesis of the given compound is shown below.
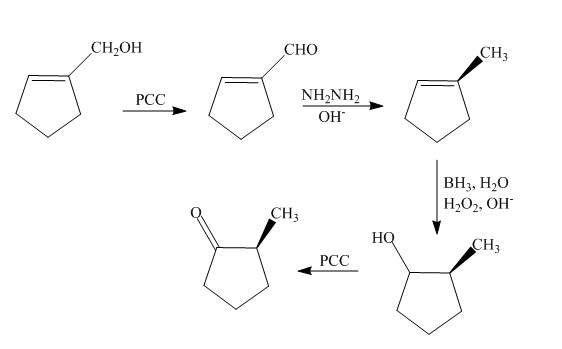
Explanation of Solution
The reaction of cyclopentenyl methanol with

Figure 12
The synthesis of the given compound is shown in Figure 12.
(m)
Interpretation:
The synthesis of the given compound is to be drawn.
Concept introduction:
The substances which on addition removes oxygen atom or hydrogen atom from the other substance, that is, reduces the other substances are known as reducing agents. Reducing agents themselves get oxidized. Strong reducing agents like
Answer to Problem 11.61AP
The synthesis of the given compound is shown below.
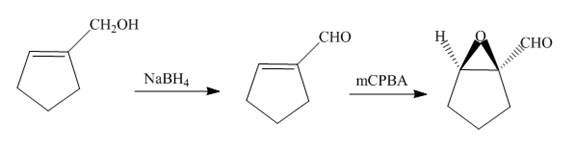
Explanation of Solution
The alcohol is reduced in presence of

Figure 13
The synthesis of the given compound is shown in Figure 13.
Want to see more full solutions like this?
Chapter 11 Solutions
ORGANIC CHEM +SG +SAPLING >IP<
- Draw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forwardWhat is the unsaturation number for compounds with the formula C₂H₁₂Cl₂? O õ õ o o 4 3arrow_forwardIndicate the product obtained (formula). F3C. CF3 Br NH2 NH OMe K2CO3, DABCO, DMFarrow_forward
- What are the missing intermediates 1, 2, and 3? Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediates and how they occur.arrow_forwardThe following intermediates are to proceed by acetoacetic ester synthesis. What are intermediates 1 and 2 plus the final product 3? Please include a detailed explanation and drawings of the intermediates and how they occurred.arrow_forwardThe chemical formula of "benzimidazole E" is C7H6N2. Draw it.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





