
The following rate constants were obtained in an experiment in which the decomposition of gaseous N2O; was studied as a function of temperature. The products were NO, and NO,.
| Temperature (K) | |
| 3.5 x 10_i | 298 |
| 2.2 x 10"4 | 308 |
| 6.8 X IO-4 | 318 |
| 3.1 x 10 1 | 328 |
Determine Etfor this reaction in kj/mol.
Interpretation:Ea should be determined in kJ/mol for the reaction of decomposition of N2O5 to NO2 and NO3 when the details of a study of that reaction as a function of temperature are given.
Concept introduction:
Rate of a reaction can be explained using either growth of products or reduction of reactants. Both give the same rate, but our concern is different.
Every reaction has activation energy. To overcome this energy sometimes we should provide energy from outside. But for some reactions, we do not have to supply energy. Ambient temperature is enough that reactions. Those reactions are called spontaneous reactions.
Answer to Problem 11.55PAE
Solution:
Ea = 140.277 kJmol−1
Given:
- Chemical reaction
N2O5→NO2+ NO3
k/s−1 |
Temperature/K |
|
3.5×10−5 |
298 |
|
2.2×10−4 |
308 |
|
6.8×10−4 |
318 |
|
3.1×10−3 |
328 |
Explanation of Solution
N2O5→NO2+ NO3
The rate of the equation for the reaction can be written as follows.
R= −k [Ν2Ο5]
- The only equation relating activation energy and rate constant is Arrhenius equation which is given below. The frequency factor doesn’t depend on the temperature.
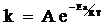
It can be written as
lnk=lnA−EaRT
Therefore, at two different temperatures at T1 and T2 ;
ln k1= ln A – EaRT1→1ln k2= ln A – EaRT2→2
When equation 1 is subtracted from equation 2,
ln (k2/k1) = EaR (1T1– 1T2)
Formula used:
ln (k2/k1) = EaR (1T1– 1T2)
Calculation:
ln (k2/k1) = EaR (1T1– 1T2) Substitution of valuesln (2.2×10−4/3.5×10−5) = Ea8.314 (1298−1308)Ea = 140.277 kJmol−1
Ea = 140.277 kJmol−1
Want to see more full solutions like this?
Chapter 11 Solutions
EBK CHEMISTRY FOR ENGINEERING STUDENTS,
- 19.57 Using one of the reactions in this chapter, give the correct starting material (A-L) needed to produce each structure (a-f). Name the type of reaction used. (b) ہ مرد (d) HO (c) དང་ ་་ཡིན་ད་དང་ (f) HO Br B D of oli H J Br K C 人 ↑arrow_forwardInductive effect (+I and -I) in benzene derivatives.arrow_forward7. Helparrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





