
EBK CHEMISTRY FOR ENGINEERING STUDENTS,
4th Edition
ISBN: 9781337671439
Author: Holme
Publisher: CENGAGE LEARNING - CONSIGNMENT
expand_more
expand_more
format_list_bulleted
Question
Chapter 11, Problem 11.109PAE
Interpretation Introduction
Interpretation:
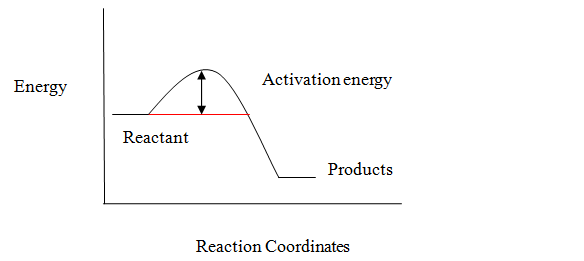
To explain why reactions with free radical are carried out at higher temperatures.
Concept introduction:
Arrhenius equation:
Very small changes in the temperature can lead to drastic changes in the
Activation energy is defined as the minimum amount of energy required to start a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the table of Reactants and Products provided in the Hints section, provide the major product
(with the correct stereochemistry when applicable) for questions below by selecting the letter that
corresponds to the exact chemical structures for the possible product.
OH conc Hydrochloric
acid
40°C Temp
A/
Using arrows to designate the flow of electrons, complete the reaction below and
provide a detailed mechanism for the formation of the product
OH
conc Hydrochloric
acid
40°C Temp
All chemical structures should be hand drawn on a piece of paper
Paragraph
BI UAE +v
draw out the following structures plese
Chapter 11 Solutions
EBK CHEMISTRY FOR ENGINEERING STUDENTS,
Ch. 11 - Prob. 1COCh. 11 - . define the rate of a chemical reaction and...Ch. 11 - Prob. 3COCh. 11 - Prob. 4COCh. 11 - . explain the difference between elementary...Ch. 11 - . find the rate law predicted for a particular...Ch. 11 - . use a molecular perspective to explain the...Ch. 11 - Prob. 8COCh. 11 - . explain the role of a catalyst in the design of...Ch. 11 - Prob. 11.1PAE
Ch. 11 - List two types of chemical compounds that must be...Ch. 11 - Prob. 11.3PAECh. 11 - Prob. 11.4PAECh. 11 - Prob. 11.5PAECh. 11 - Prob. 11.6PAECh. 11 - Asphalt is composed of a mixture of organic...Ch. 11 - Prob. 11.8PAECh. 11 - Prob. 11.9PAECh. 11 - For each of the following, suggest appropriate...Ch. 11 - Prob. 11.11PAECh. 11 - Rank the following in order of increasing reaction...Ch. 11 - Prob. 11.13PAECh. 11 - Candle wax is a mixture of hydrocarbons. In the...Ch. 11 - Prob. 11.15PAECh. 11 - The reaction for the Haber process, the industrial...Ch. 11 - 11.17 Ammonia can react with oxygen to produce...Ch. 11 - The following data were obtained in the...Ch. 11 - Prob. 11.19PAECh. 11 - Experimental data are listed here for the reaction...Ch. 11 - Azomethane, CH3NNCH3, is not a stable compound,...Ch. 11 - Prob. 11.22PAECh. 11 - A reaction has the experimental rate equation Rate...Ch. 11 - Second-order rate constants used in modeling...Ch. 11 - For each of the rate laws below, what is the order...Ch. 11 - 11.26 The reaction of C(Xg) with NO2(g) is second...Ch. 11 - Prob. 11.27PAECh. 11 - Prob. 11.28PAECh. 11 - The hypothetical reaction, A + B —*C, has the rate...Ch. 11 - The rate of the decomposition of hydrogen...Ch. 11 - Prob. 11.31PAECh. 11 - 11.32 The following experimental data were...Ch. 11 - The following experimental data were obtained for...Ch. 11 - 11.34 Rate data were obtained at 25°C for the...Ch. 11 - 11.35 For the reaction 2 NO(g) + 2 H?(g) — N,(g) +...Ch. 11 - The reaction NO(g) + O,(g) — NO,(g) + 0(g) plays a...Ch. 11 - Prob. 11.37PAECh. 11 - Prob. 11.38PAECh. 11 - The decomposition of N2O5 in solution in carbon...Ch. 11 - In Exercise 11.39, if the initial concentration of...Ch. 11 - 11.41 For a drug to be effective in treating an...Ch. 11 - Amoxicillin is an antibiotic packaged as a powder....Ch. 11 - As with any drug, aspirin (acetylsalicylic acid)...Ch. 11 - 11.44 A possible reaction for the degradation of...Ch. 11 - The initial concentration of the reactant in a...Ch. 11 - A substance undergoes first-order decomposition....Ch. 11 - Prob. 11.47PAECh. 11 - 11.48 The following data were collected for the...Ch. 11 - The rate of photodecomposition of the herbicide...Ch. 11 - Prob. 11.50PAECh. 11 - 11.51 Peroxyacetyl nitrate (PAN) has the chemical...Ch. 11 - Hydrogen peroxide (H20i) decomposes into water and...Ch. 11 - 11.53 The reaction in which CO, decomposes to CO...Ch. 11 - use the kineticmolecular theory to explain why an...Ch. 11 - The following rate constants were obtained in an...Ch. 11 - The table below presents measured rate constants...Ch. 11 - Prob. 11.57PAECh. 11 - Prob. 11.58PAECh. 11 - Can a reaction mechanism ever be proven correct?...Ch. 11 - Prob. 11.60PAECh. 11 - Describe how the Chapman cycle is a reaction...Ch. 11 - Prob. 11.62PAECh. 11 - The following mechanism is proposed for a...Ch. 11 - 11.64 HBr is oxidized in the following reaction: 4...Ch. 11 - Prob. 11.65PAECh. 11 - Prob. 11.66PAECh. 11 - What distinguishes homogeneous and heterogeneous...Ch. 11 - Prob. 11.68PAECh. 11 - In Chapter 3, we discussed the conversion of...Ch. 11 - The label on a bottle of 3% (by volume) hydrogen...Ch. 11 - Prob. 11.71PAECh. 11 - Prob. 11.72PAECh. 11 - Prob. 11.73PAECh. 11 - 11.74 The AQI includes six levels, including...Ch. 11 - Prob. 11.75PAECh. 11 - Prob. 11.76PAECh. 11 - Prob. 11.77PAECh. 11 - Prob. 11.78PAECh. 11 - Prob. 11.79PAECh. 11 - Prob. 11.80PAECh. 11 - Prob. 11.81PAECh. 11 - Prob. 11.82PAECh. 11 - Bacteria cause milk to go sour by generating...Ch. 11 - Prob. 11.84PAECh. 11 - Prob. 11.85PAECh. 11 - Prob. 11.86PAECh. 11 - Prob. 11.87PAECh. 11 - Prob. 11.88PAECh. 11 - Prob. 11.89PAECh. 11 - 11.90 Draw a hypothetical activation energy...Ch. 11 - Prob. 11.91PAECh. 11 - Prob. 11.92PAECh. 11 - 11.93 On a particular day, the ozone level in...Ch. 11 - Prob. 11.94PAECh. 11 - The following is a thought experiment. Imagine...Ch. 11 - The following statements relate to the reaction...Ch. 11 - Prob. 11.97PAECh. 11 - Experiments show that the reaction of nitrogen...Ch. 11 - Substances that poison a catalyst pose a major...Ch. 11 - Prob. 11.100PAECh. 11 - Prob. 11.101PAECh. 11 - 11.102 Suppose that you are studying a reaction...Ch. 11 - Prob. 11.103PAECh. 11 - Prob. 11.104PAECh. 11 - Prob. 11.105PAECh. 11 - Prob. 11.106PAECh. 11 - 11.1047 Fluorine often reacts explosively. What...Ch. 11 - Prob. 11.108PAECh. 11 - Prob. 11.109PAECh. 11 - When formic acid is heated, it decomposes to...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw everything on a piece of paper outlining the synthesis from acetaldehyde to 2 cyclopentene carboxaldehyde using carbon based reagants with 3 carbons or fewers. Here is the attached image.arrow_forwardManoharan Mariappan, FR.D., 34) Complete the following reaction starting from hex-1-yne proceeding via different substitution reactions forming 2-heptanone. (25 pts). A Sia₂BH H₂O₂ NaOH Br D Mechanism for reaction D - ether-cleavage: 10 B Ph-MgCI, THF H₁₂O+ D HBr (XS) C TsCl, Py CH3-CH2-CH2-ONaarrow_forwardIn the table below, the correct structure for (2R)-3-methylpentan-2-ol (IUPAC name) can be represented by the letter OH OH HE > ' ÕH C B OH D A/ E OHarrow_forward
- Predict the major products of the following organic reaction: + A Δ ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Check Click and drag to start drawing a structure. Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forwardWhy is analysing salt content (using Mohr titration) in both regular & salt reduced tomato sauce important?arrow_forwardIn the image below, correctly name the glassware # _P ( Blank 1) and T ( Blank 2). 景 A W Blank # 1 Blank #2 1000 +19 E E D 0 0-0 G H A A K Π 12 R M N S 0-0-arrow_forward
- Feedback: Your answer is incorrect. Predict the major products of the following organic reaction: CN Δ + A ? NC Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. esc Check 80 MH F1 F2 F3 F4 F5 50 @ # C % 95 € Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C A DII F6 F7 F8 7 * 8 Λ & 6 F9 F10 9 0 4arrow_forwardIncorrect Feedback: Your answer is incorrect. Predict the major products of the following organic reaction: ཤིགས་བྱ རྩ་ཅད་ཀྱིས་༢༩ + Some important notes: A ^ ? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. E Check 0 لا Save For La ©2025 McGraw Hill LLC. All Rights Reserved. Terms of All F9 Aarrow_forwardPredict the major products of the following organic reaction: + Δ A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privaarrow_forward
- esc 2 Incorrect Feedback: Your answer is incorrect. Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? A O • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. . If your answer is no, check the box under the drawing area instead. Check F1 ! @ X C Save For Later Submit Assignment 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 80 et A ད 1 4 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 # $ 45 % A 6 87 & * 8 9 ) 0 + ||arrow_forwardCan the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ?A Δ O • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilit ku F11arrow_forward१ eq ine teaching and × + rn/takeAssignment/takeCovalentActivity.do?locator-assignment-take [Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is In progress mit Answer Retry Entire Group 5 more group attempts remaining Cengage Learning | Cengage Technical Support Save and Exitarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Kinetics: Initial Rates and Integrated Rate Laws; Author: Professor Dave Explains;https://www.youtube.com/watch?v=wYqQCojggyM;License: Standard YouTube License, CC-BY