
Concept explainers
(a)
Interpretation:
The major product of the given electrophilic addition reaction is to be predicted.
Concept introduction:
Strong Bronsted acids add to the double bond in a two-step electrophilic addition reaction to replace the
Answer to Problem 11.52P
The major product of the given reaction is
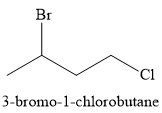
Explanation of Solution
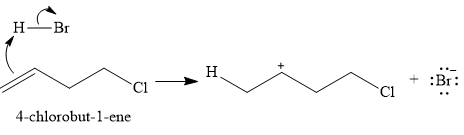
The given reaction is
The substrate is an
In the first step, the
In the second step, the conjugate base
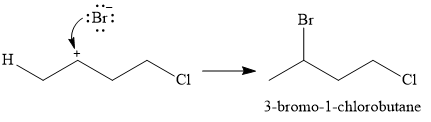
Thus, for the given reaction, the major product formed is

Strong Bronsted acids add to a double bond, replacing the
(b)
Interpretation:
The major product of the given electrophilic addition reaction is to be predicted.
Concept introduction:
In a two-step electrophilic addition reaction, the strong Bronsted acids add to the double bond to replace the
Answer to Problem 11.52P
For the given reaction, the major product formed is,
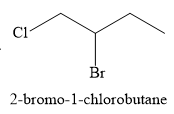
Explanation of Solution
The given electrophilic addition reaction is
The first step of the reaction is electrophilic addition of the proton from the acid. Two carbocations are possible when the
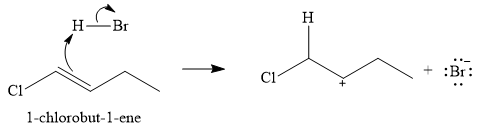
The conjugate base
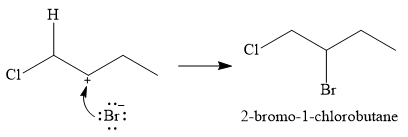
Thus, for this reaction, the major product formed is,

Strong Bronsted acids add to a double bond, replacing the
(c)
Interpretation:
The major product of the given electrophilic addition reaction is to be predicted.
Concept introduction:
In a two-step electrophilic addition reaction, the strong Bronsted acids add to the double bond to replace the
Answer to Problem 11.52P
For this reaction, the major product formed is,
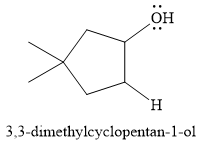
Explanation of Solution
The given reaction is
The overall reaction is one of hydration of the double bond, i.e., the addition of water. Water is a weak acid, but in the presence of a strong acid (denoted by the
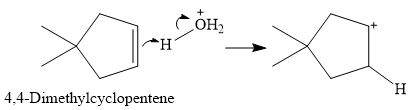
In the next step, the conjugate base
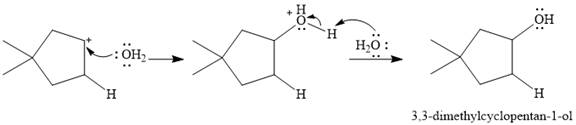
Thus, for this reaction, the major product formed is,

Strong Bronsted acids add to a double bond, replacing the
(d)
Interpretation:
The major product of the given electrophilic addition reaction is to be predicted.
Concept introduction:
In a two-step electrophilic addition reaction, the strong Bronsted acids add to the double bond to replace the
Answer to Problem 11.52P
For the given reaction, the major product formed is,
Explanation of Solution
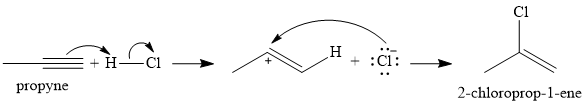
The given reaction is
There are two
In the first step, an alkenyl carbocation is formed by addition of proton the triple bond. A more stable carbocation, with the positive charge on the secondary carbon, is produced.

In the second step, the conjugate base
Since the product has a
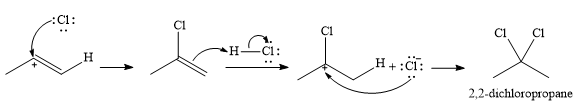
Thus, for this reaction, the major product formed is,

Strong Bronsted acids add twice to a triple bond, replacing each
(e)
Interpretation:
The major product of the given electrophilic addition reaction is to be predicted.
Concept introduction:
In a two-step electrophilic addition reaction, the strong Bronsted acids add to the double bond to replace the
Answer to Problem 11.52P
For the given reaction, the major product formed is,
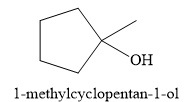
Explanation of Solution
The given electrophilic addition reaction is
![]()
The acid in this case is
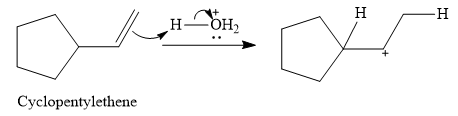
This carbocation is prone to rearrangement. A
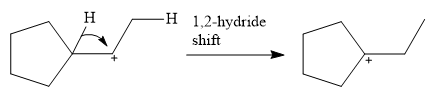
The conjugate base
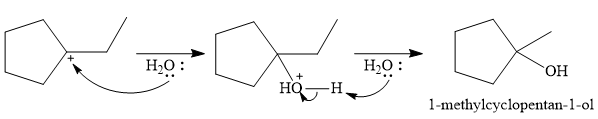
Thus, for the given reaction, the major product formed is,

Strong Bronsted acids add to a double bond, replacing the
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry: Principles And Mechanisms
- For questions 1-4, consider the following complexes: [Co(CN)6], [COC14]², [Cr(H2O)6]²+ 4. Room temperature (20°C) measurement of molar magnetic susceptibility (Xm) for Fe(NH4)2(SO4)2×6H2O is 1.1888 x 102 cgs (Gaussian units). Calculate effective magnetic moment and provide a number of unpaired electrons for the iron ion. Use this number to rationalize the coordination geometry around iron center. (4 points)arrow_forward7. Describe the expected 31P and 19F (where applicable) NMR spectral patterns for the following compounds (indicate number of signals and their splitting patterns). a) tetraphenyldiphosphine Ph Ph P-P Ph Ph Ph Ph ' b) tetraphenyldiphosphine monoxide P-P-Ph Ph (2 points) (2 points c) tetrafluorophosphonium hexafluorophosphate [PF4]*[PF6]¯ (4 points)arrow_forward3. For questions 1-4, consider the following complexes: [Co(CN)6]4, [COC14]², [Cr(H2O)6]²+ Which (if any) of these complexes would be expected to display Jahn-Teller distortion? (2 points)arrow_forward
- What is Instrumental Neutron Activation and what are the advantages and disadvantages in using its applications? (I'm doing an in class assignment and need better understanding of what the instrument can be used for) Please include references so that I can better understand the application of how the instrument works!arrow_forwardWhat is Isotope Analysis and what are the advantages and disadvantages in using its applications and instrumentalization? Please include references so that I can better understand how the instrument works!arrow_forward5. Count the electrons on the following complexes and state whether they follow the 18- electron rule: (3 points) Fe(CO)5 Ni(PMe3)4 PMe3 is trimethylphosphine Mn(CO)5Brarrow_forward
- For questions 1-4, consider the following complexes: [Co(CN)6]+, [CoCl4]², [Cr(H2O)6]²+ 2. Draw the corresponding d-orbital splitting for each of the complexes; predict the spin- state (low-spin/high spin) for each of the complexes (if applicable); explain your arguments. Calculate the crystal field stabilization energy for each complex (in Ao or At). (6 points)arrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6]4, [COC14]², [Cr(H2O)6]²+ 1. Assign oxidation number to the metal, then indicate d-electron count. (3 points)arrow_forwardUsing iodometry I want to titrate a sodium thiosulfate solution and I use 15 mL. If I have 50 mL of a 0.90 M copper solution and KI, what will be the molarity of sodium thiosulfate?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

