
(a)
Interpretation:
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom in
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
The partial orbital diagram is the one that shows the distribution of electrons in the valence shell only.
(a)
Answer to Problem 11.41P
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom iodine in
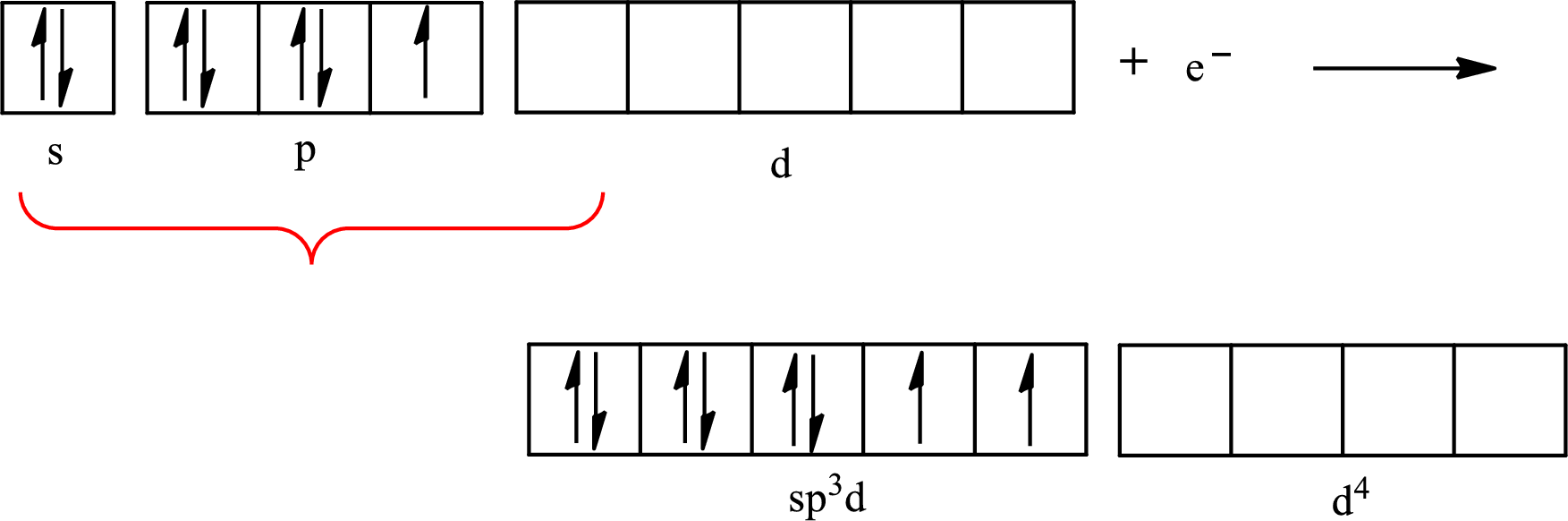
Explanation of Solution
The Lewis structure of
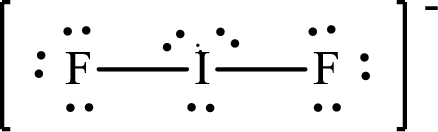
Iodine forms two single bonds with two fluorine atoms and three lone pairs are present on it so the hybridization of iodine in
The
The partial orbital diagram for an isolated
The partial orbital for hybridized
One s orbital, three p orbitals and one d orbital of central atom iodine combine to form five
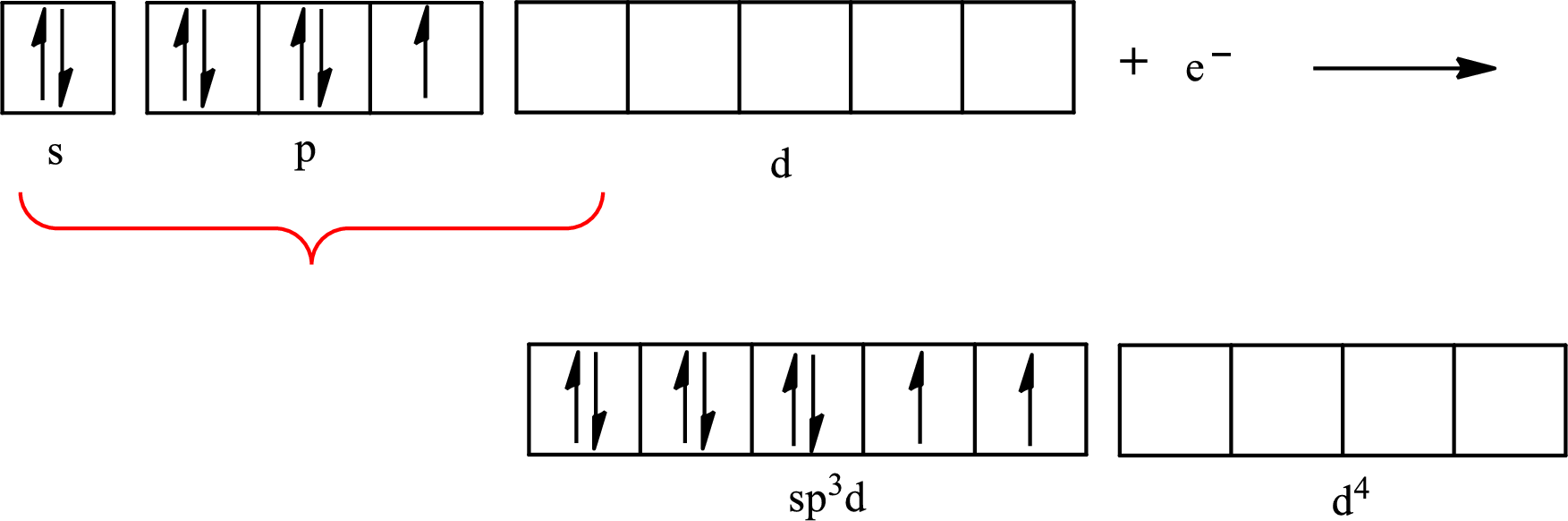
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom iodine in
(b)
Interpretation:
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom in
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
The partial orbital diagram is the one that shows the distribution of electrons in the valence shell only.
(b)
Answer to Problem 11.41P
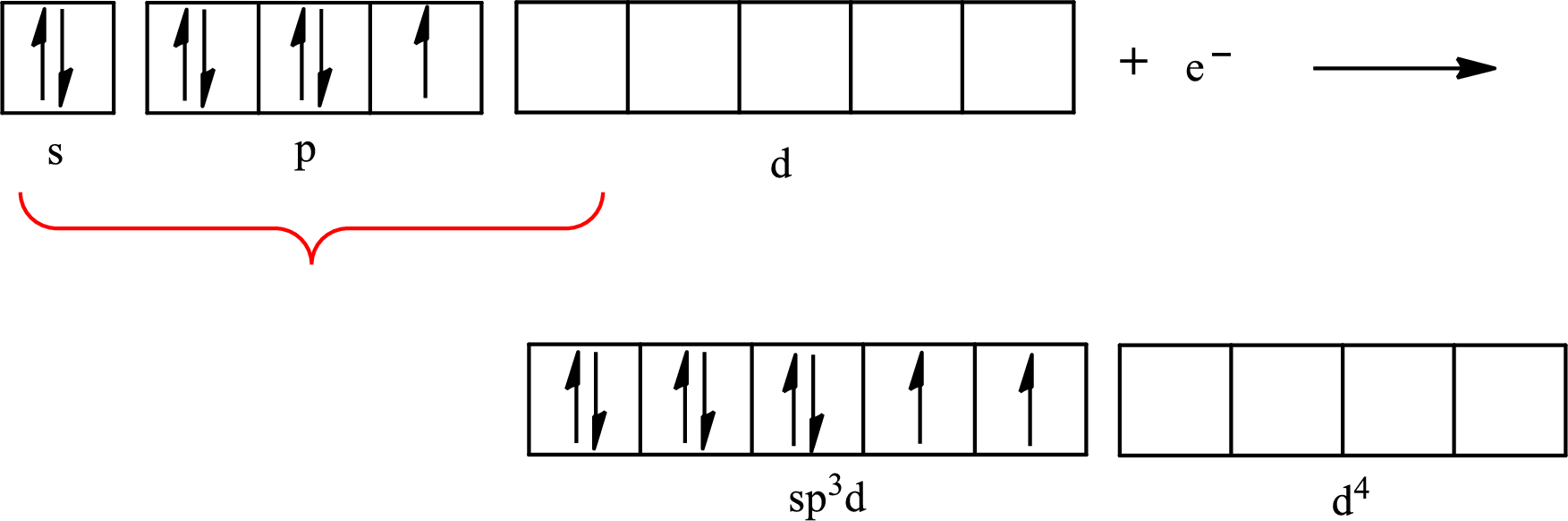
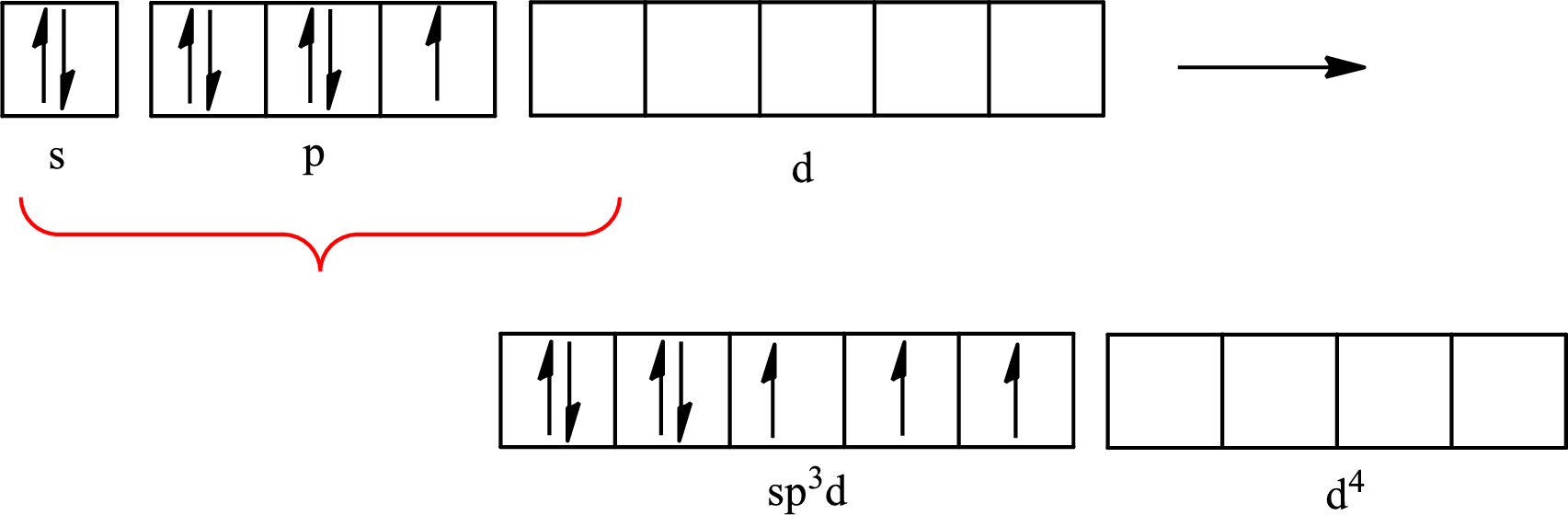
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom iodine in
Explanation of Solution
The Lewis structure of
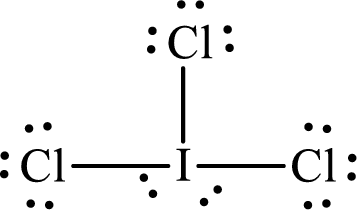
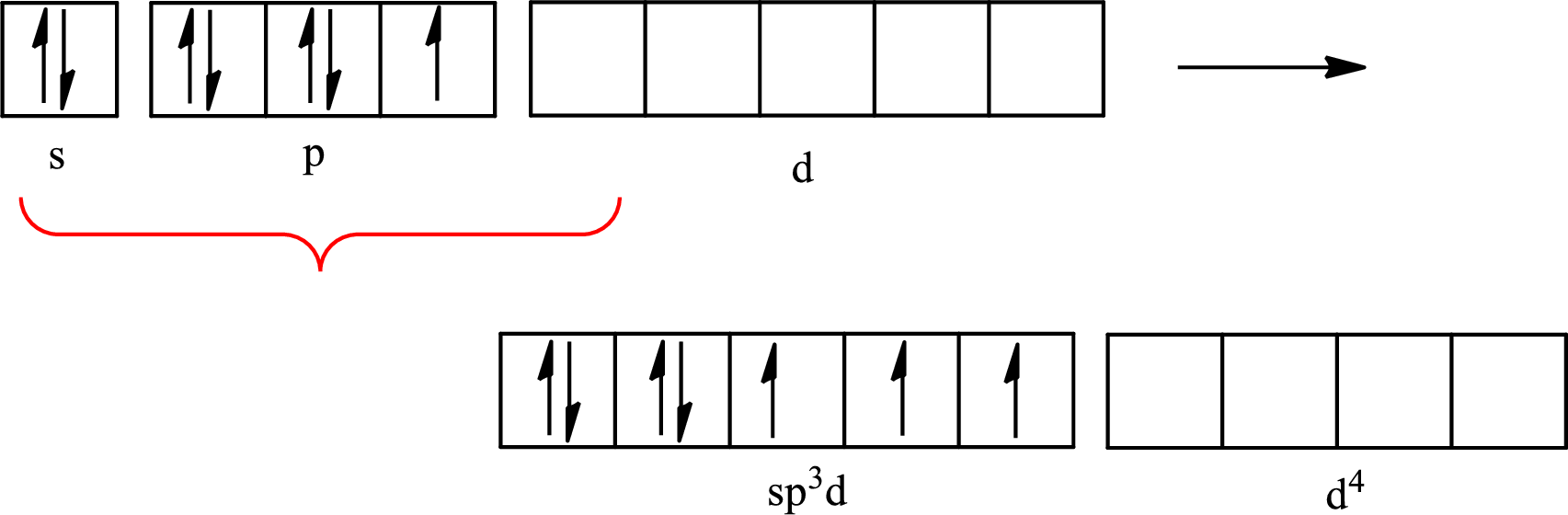
Iodine forms three single bonds with three chlorine atoms and two lone pairs are present on it so five hybrid orbitals are required. The hybridization of
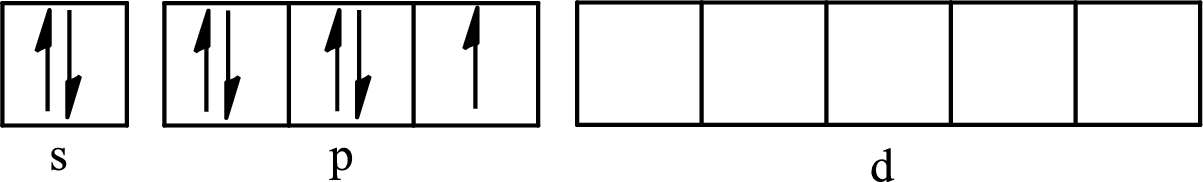
The atomic number of iodine is 53 so its electronic configuration is
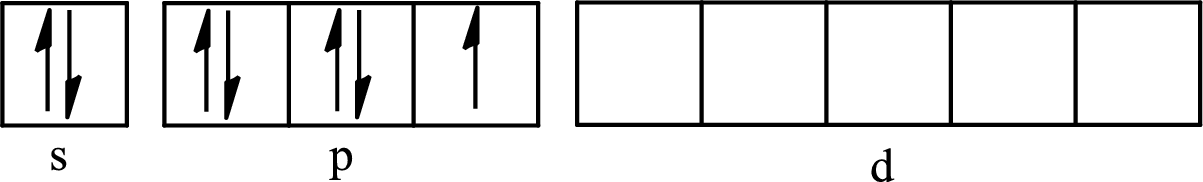
The partial orbital diagram for an isolated
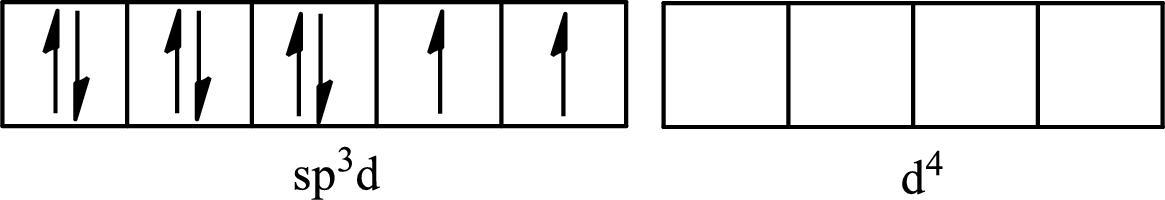
The partial orbital for hybridized
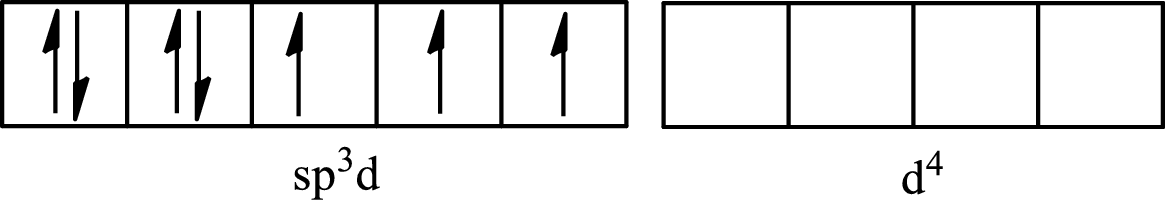
One s orbital, three p orbitals and one d orbital of central atom iodine combine to form five
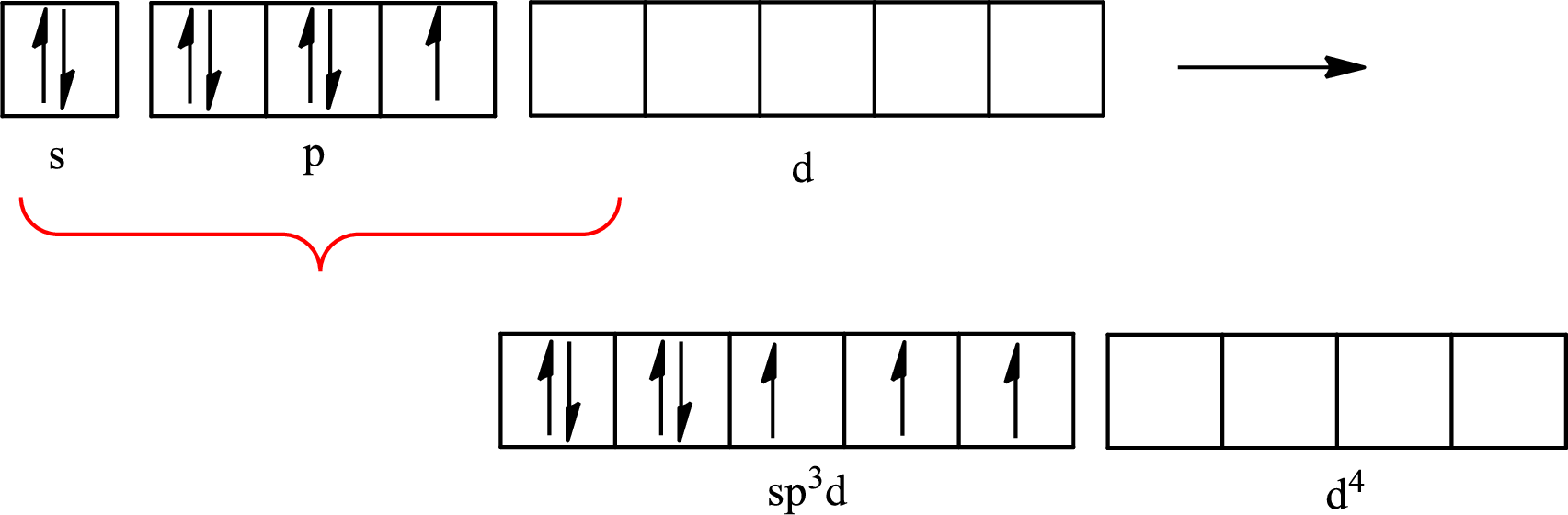
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom iodine in
(c)
Interpretation:
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom in
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
The partial orbital diagram is the one that shows the distribution of electrons in the valence shell only.
(c)
Answer to Problem 11.41P
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom xenon in
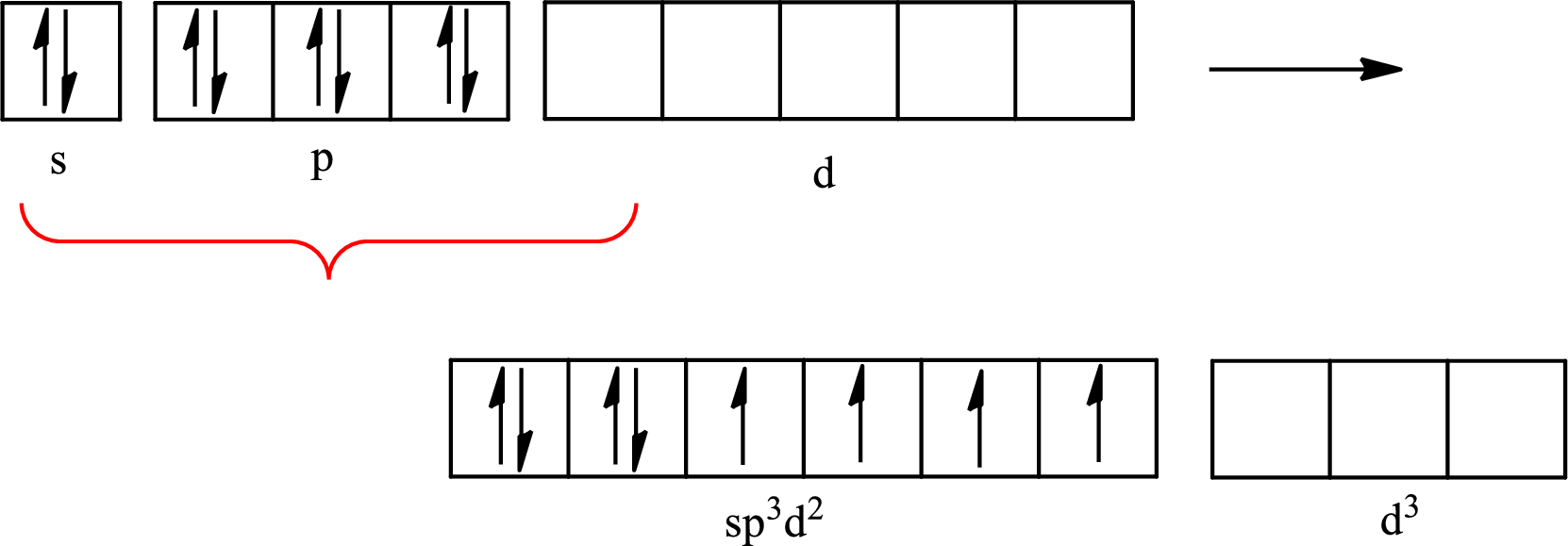
Explanation of Solution
The Lewis structure of
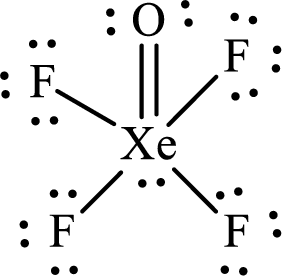
Xenon forms four single bonds with four fluorine atoms and one double bond with oxygen and one lone pair is present on it so six hybrid orbitals are required. The hybridization of xenon in
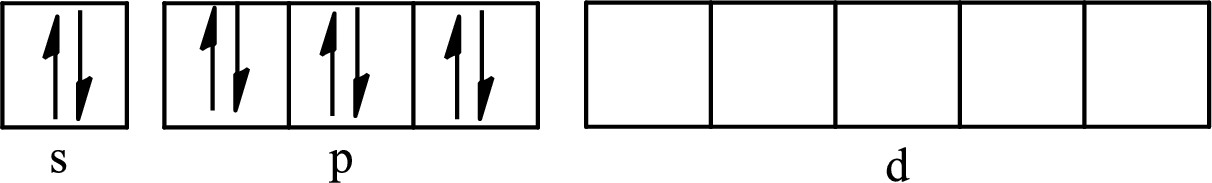
The atomic number of xenon is 54 so its electronic configuration is
The partial orbital diagram for an isolated
The partial orbital for hybridized
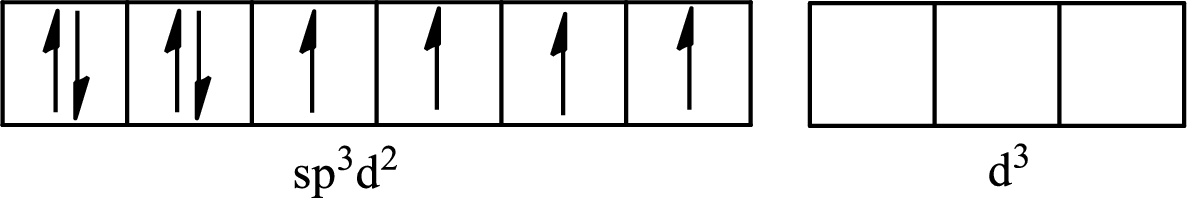
One s orbital, three p orbitals and two d orbitals of central atom xenon combine to form six
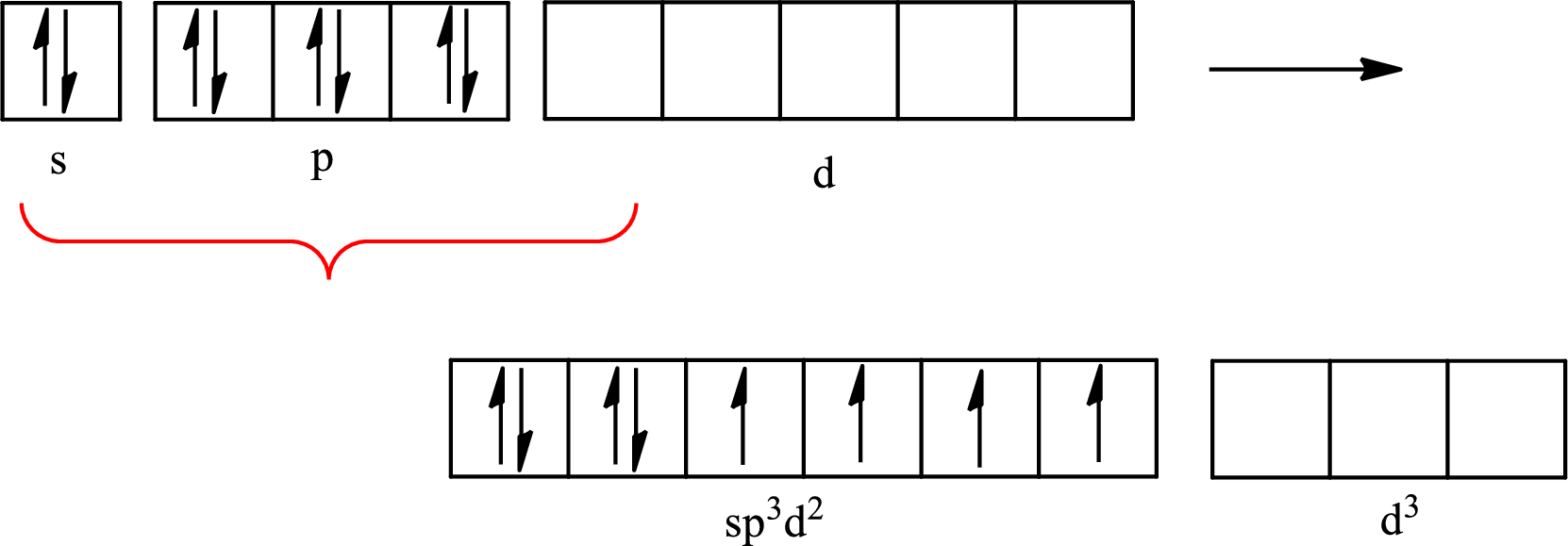
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom xenon in
(d)
Interpretation:
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom in
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
The partial orbital diagram is the one that shows the distribution of electrons in the valence shell only.
(d)
Answer to Problem 11.41P
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom boron in
Explanation of Solution
The Lewis structure of
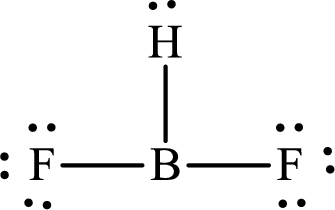
Boron forms one single bond with hydrogen and two single bonds with two fluorine atoms so three hybrid orbitals are required. The hybridization of boron in
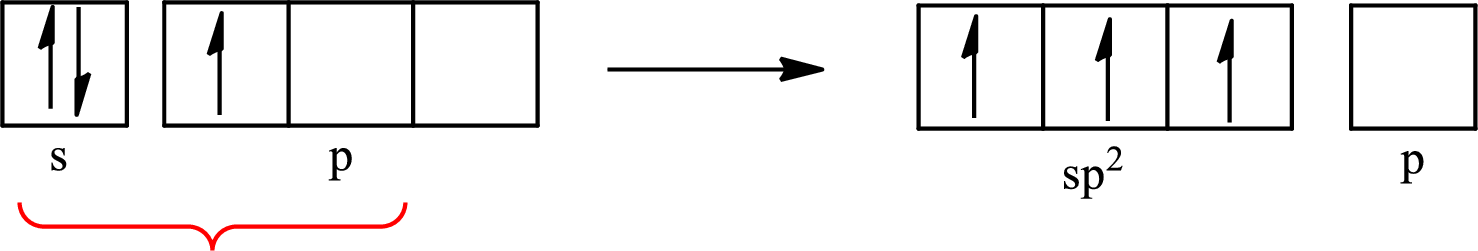
The atomic number of boron is 5 so its electronic configuration is
The partial orbital diagram for an isolated
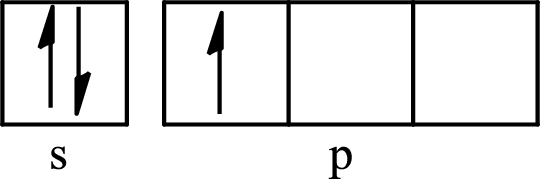
The partial orbital for hybridized
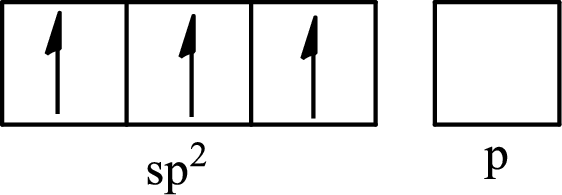
One s orbital and two p orbitals of central atom boron combine to form three
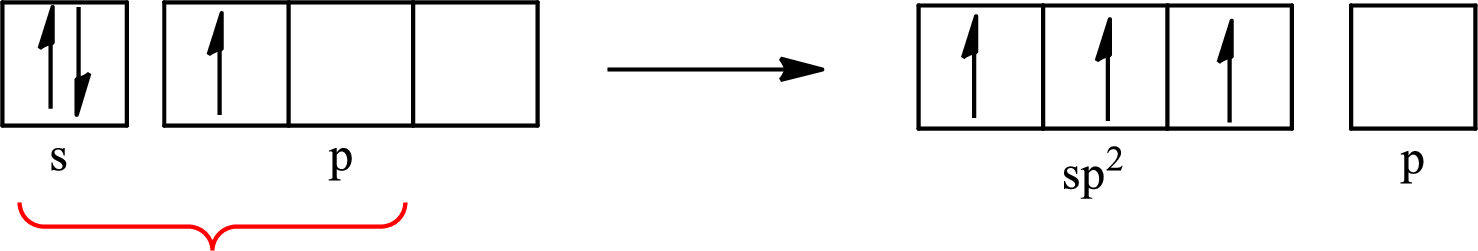
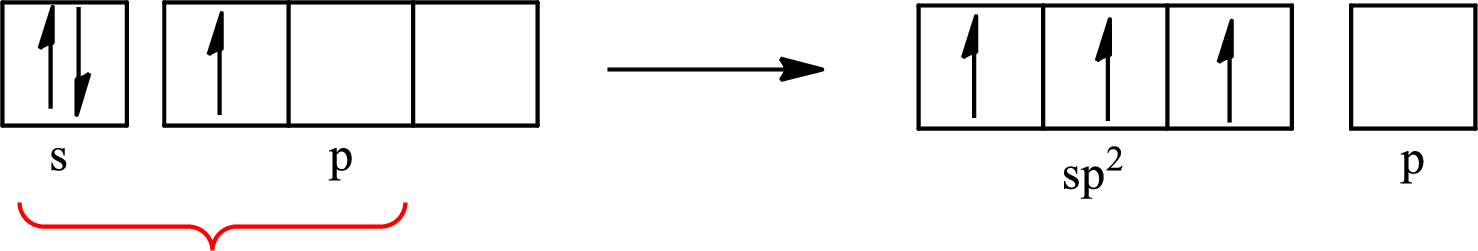
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom boron in
Want to see more full solutions like this?
Chapter 11 Solutions
Loose Leaf for Chemistry: The Molecular Nature of Matter and Change
- answer thisarrow_forwardplease add appropriate arrows and tell me in detail where to add which or draw itarrow_forwardPart 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forward
- can you please answer both these questions and draw the neccesaryarrow_forwardcan you please give the answer for both these pictures. thankyouarrow_forwardPart 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) | Bakelite like polymer Using: Resorcinol + NaOH + Formalinarrow_forward
- Question 19 0/2 pts 3 Details You have a mixture of sodium chloride (NaCl) and potassium chloride (KCl) dissolved in water and want to separate out the Cl- ions by precipitating them out using silver ions (Ag+). The chemical equation for the net ionic reaction of NaCl and KCl with silver nitrate, AgNO3, is shown below. Ag+(aq) + Cl(aq) → AgCl(s) The total mass of the NaCl/KCl mixture is 1.299 g. Adding 50.42 mL of 0.381 M solution precipitates out all of the Cl-. What are the masses of NaCl and KCl in the mixture? Atomic masses: g: Mass of NaCl g: Mass of KCL Ag = 107.868 g mol- 1 Cl = 35.453 g mol- 1 K = 39.098 g mol- N = 14.007 g mol−1 Na = 22.99 g mol−1 0 = 15.999 g mol 1 Question Help: ✓ Message instructor Submit Questionarrow_forwardPart 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Polyester fiber Using a) pthalic anhydride + anhydrous sodium acetate + ethylene glycol B)pthalic anhydride + anhydrous sodium acetate + glycerolarrow_forwardIdentify the missing starting materials/ reagents/ products in the following reactions. Show the stereochemistry clearly in the structures, if any. If there is a major product, draw the structures of the major product with stereochemistry clearly indicated where applicable. Show only the diastereomers (you do not have to draw the pairs of enantiomers). If you believe that multiple products are formed in approximately equal amounts (hence neither is the major product), draw the structures of the products, and show the detailed mechanism of these reactions to justify the formation of the multiple products. If you believe no product is formed, explain why briefly. (6 mark for each, except f and g, which are 10 mark each)arrow_forward
- 3. What starting material would you use to synthesize 3-hydroxypentanoic acid using a NaBH4 reduction?arrow_forward1. Give stereochemical (Fischer projection) formulas for all (but no extras) the stereoisomers that could theoretically form during the reduction of a. the carbonyl group of 2-methyl-3--pentanone b. both carbonyl groups of 2,4-pentanedione (careful!) 2. Predict the products of the reduction of O=CCH2CH2CH2C=O with a. LiAlH4 b. NaBH4 CH3 OHarrow_forwardWhich of the following compounds can be synthesized using one reaction from any alkene, as a major product? If it can be synthesized, propose a route, and you may use any other starting materials, reagents and solvents as needed. If you do not think that it can be synthesized as a major product from an alkene, explain in detail why.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





