
(a)
Interpretation:
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom in
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
The partial orbital diagram is the one that shows the distribution of electrons in the valence shell only.
(a)
Answer to Problem 11.41P
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom iodine in
Explanation of Solution
The Lewis structure of
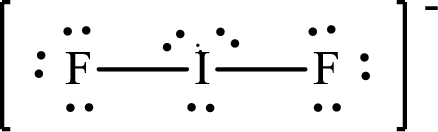
Iodine forms two single bonds with two fluorine atoms and three lone pairs are present on it so the hybridization of iodine in
The
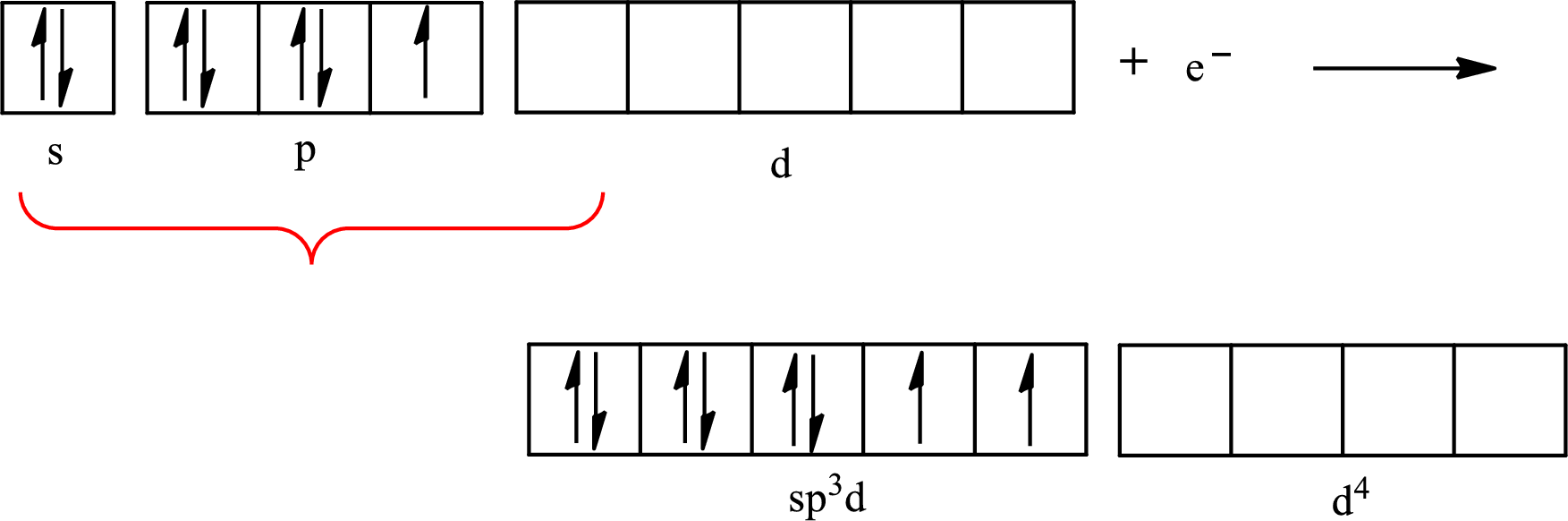
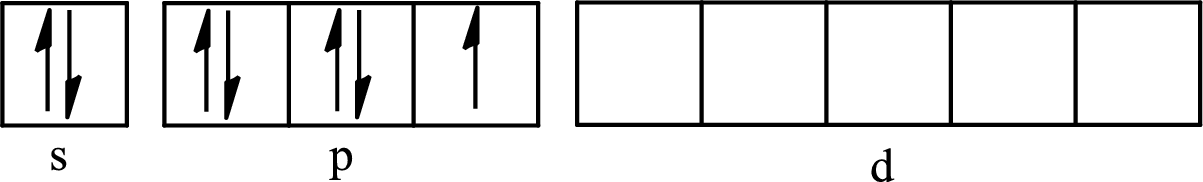
The partial orbital diagram for an isolated
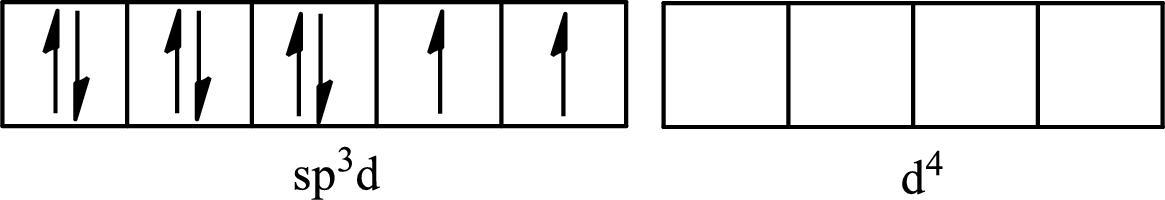
The partial orbital for hybridized
One s orbital, three p orbitals and one d orbital of central atom iodine combine to form five
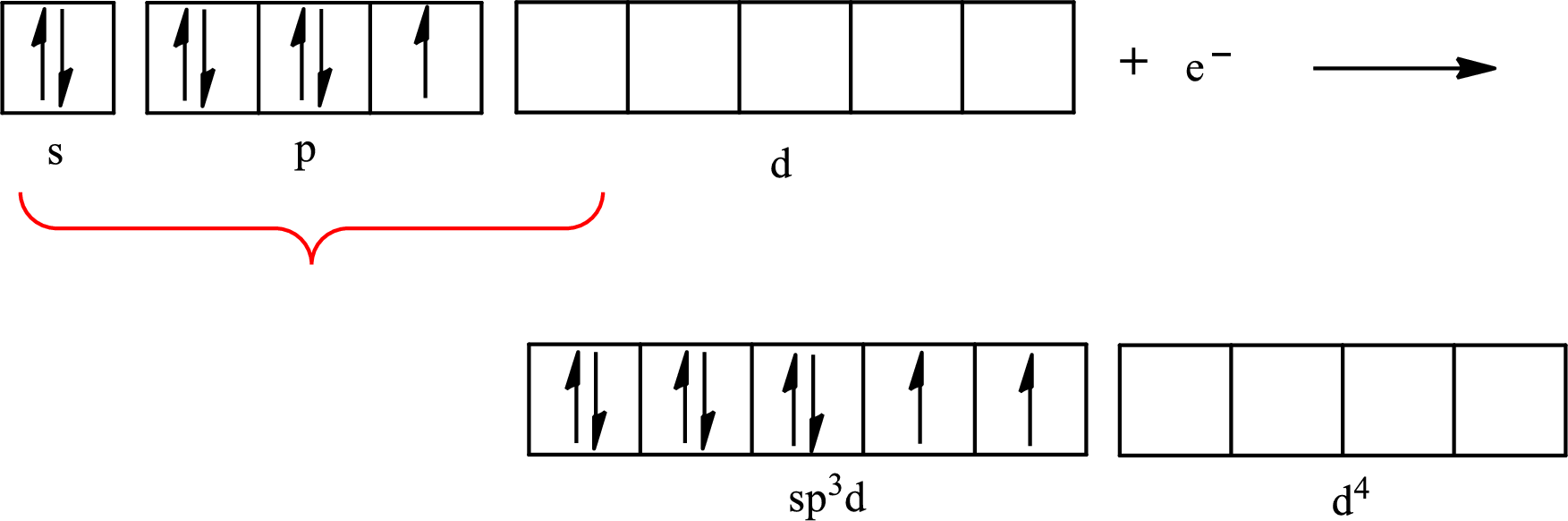
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom iodine in
(b)
Interpretation:
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom in
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
The partial orbital diagram is the one that shows the distribution of electrons in the valence shell only.
(b)
Answer to Problem 11.41P
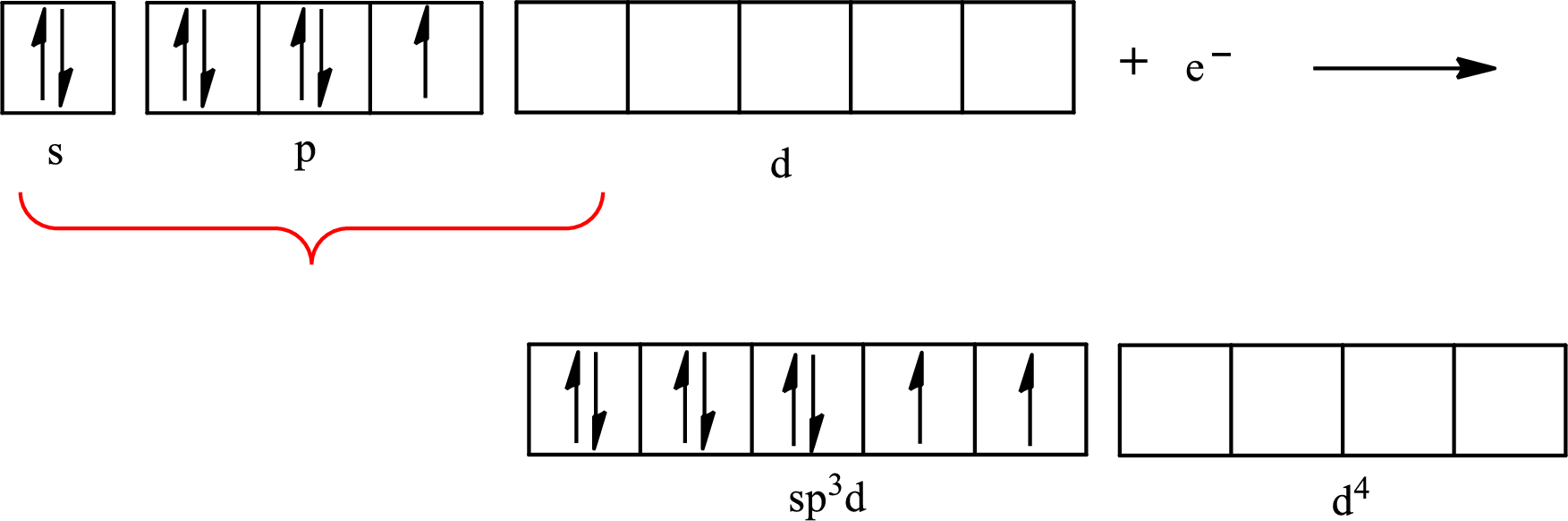
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom iodine in
Explanation of Solution
The Lewis structure of
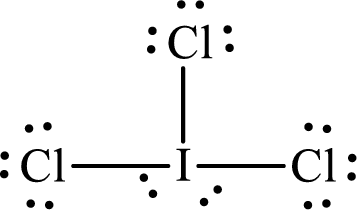
Iodine forms three single bonds with three chlorine atoms and two lone pairs are present on it so five hybrid orbitals are required. The hybridization of
The atomic number of iodine is 53 so its electronic configuration is
The partial orbital diagram for an isolated
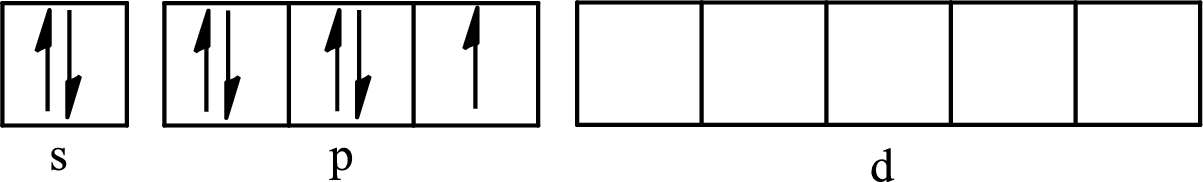
The partial orbital for hybridized
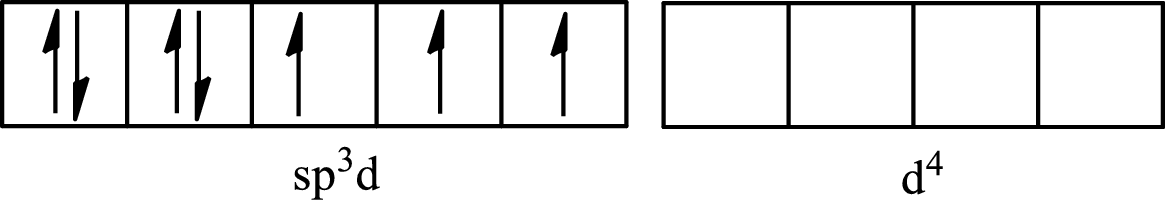
One s orbital, three p orbitals and one d orbital of central atom iodine combine to form five
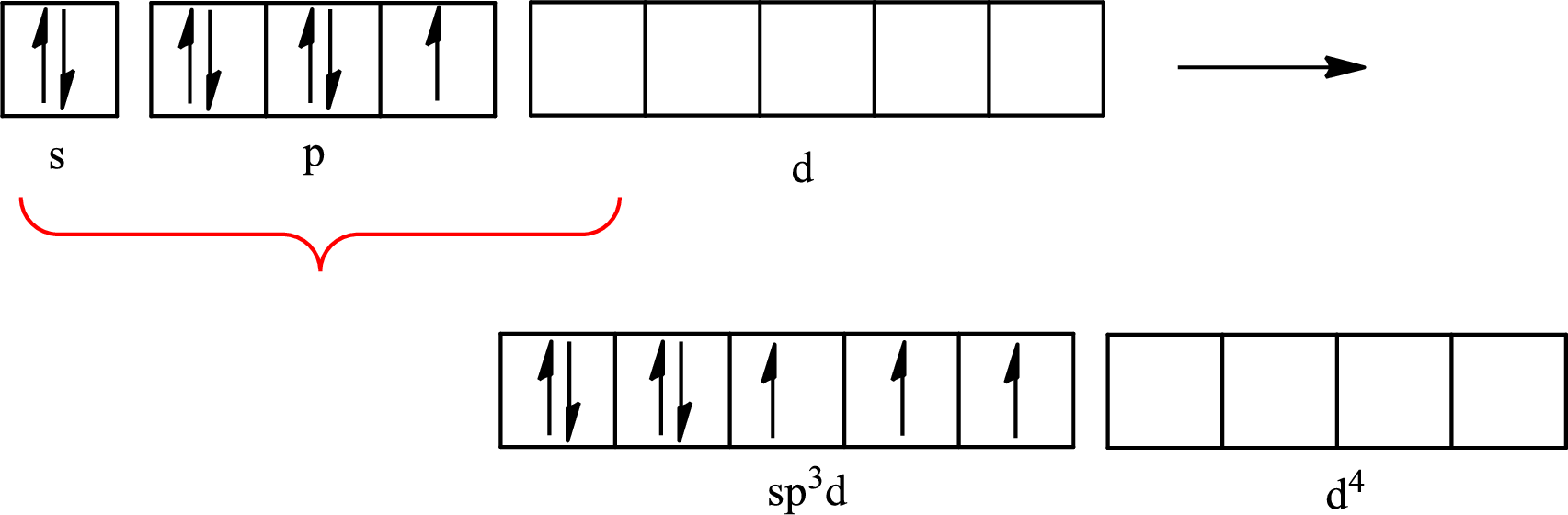
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom iodine in
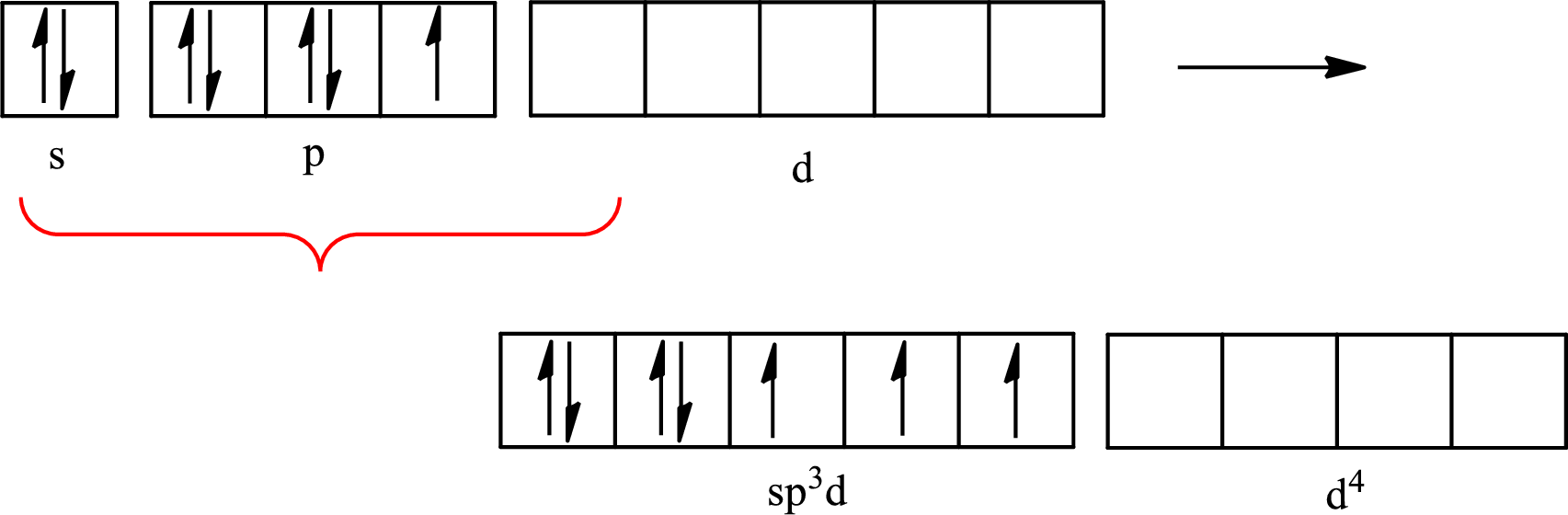
(c)
Interpretation:
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom in
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
The partial orbital diagram is the one that shows the distribution of electrons in the valence shell only.
(c)
Answer to Problem 11.41P
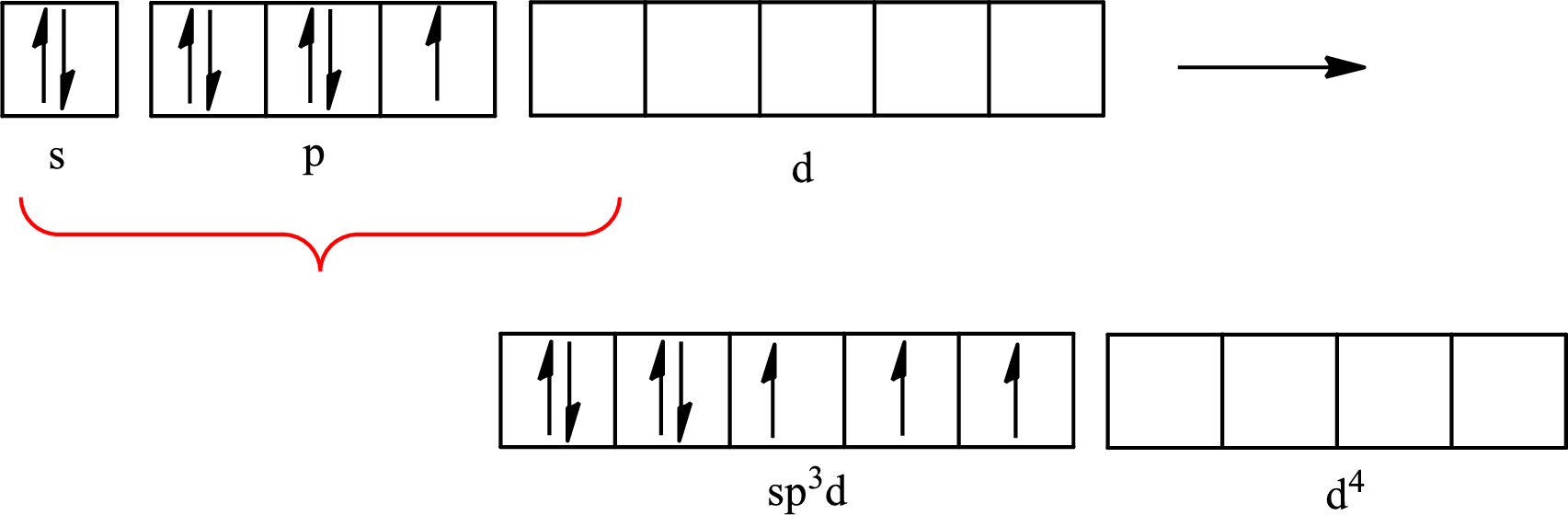
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom xenon in
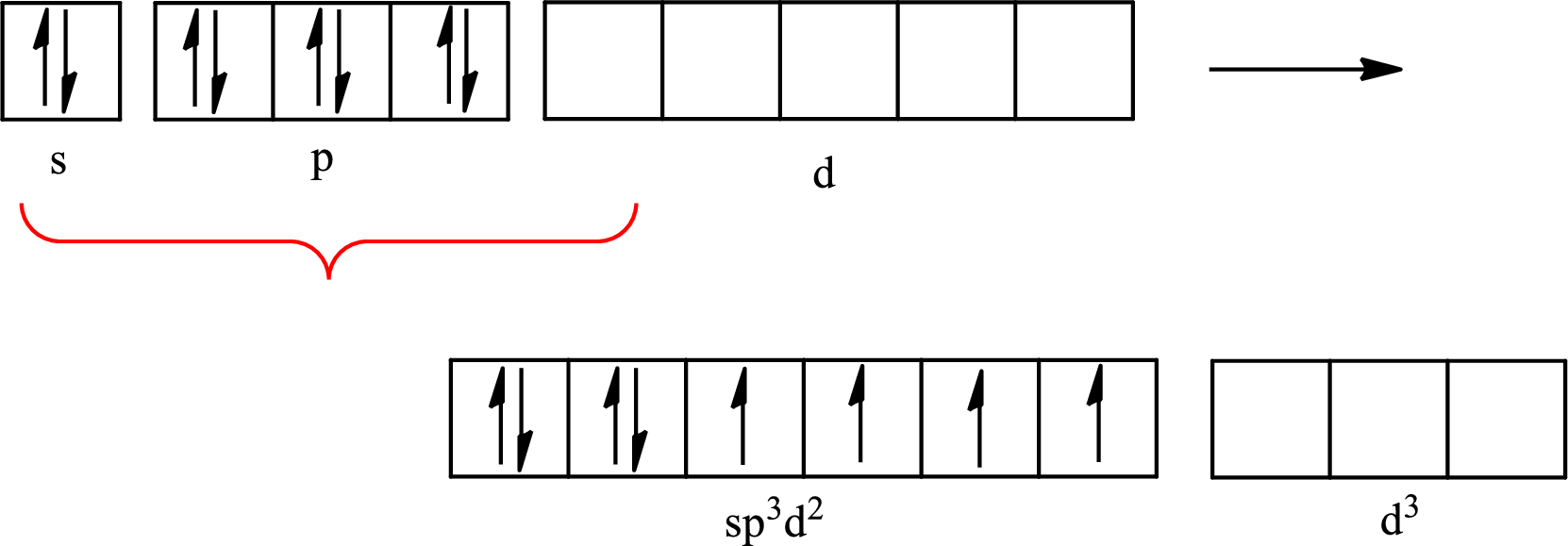
Explanation of Solution
The Lewis structure of
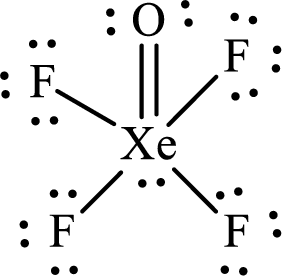
Xenon forms four single bonds with four fluorine atoms and one double bond with oxygen and one lone pair is present on it so six hybrid orbitals are required. The hybridization of xenon in
The atomic number of xenon is 54 so its electronic configuration is
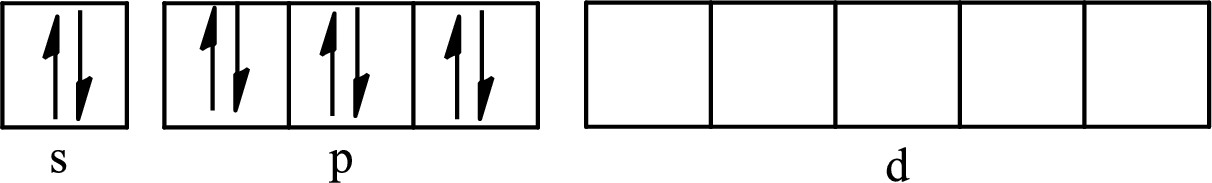
The partial orbital diagram for an isolated
The partial orbital for hybridized
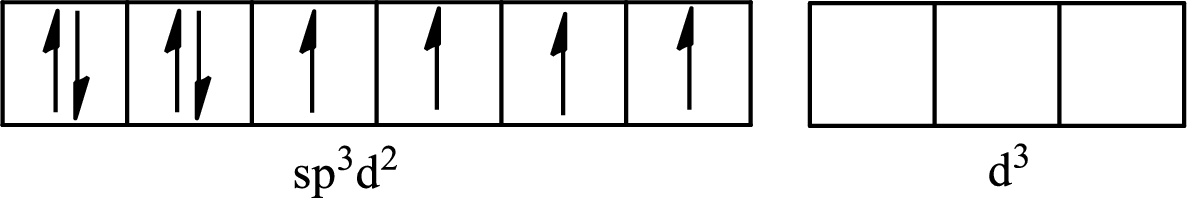
One s orbital, three p orbitals and two d orbitals of central atom xenon combine to form six
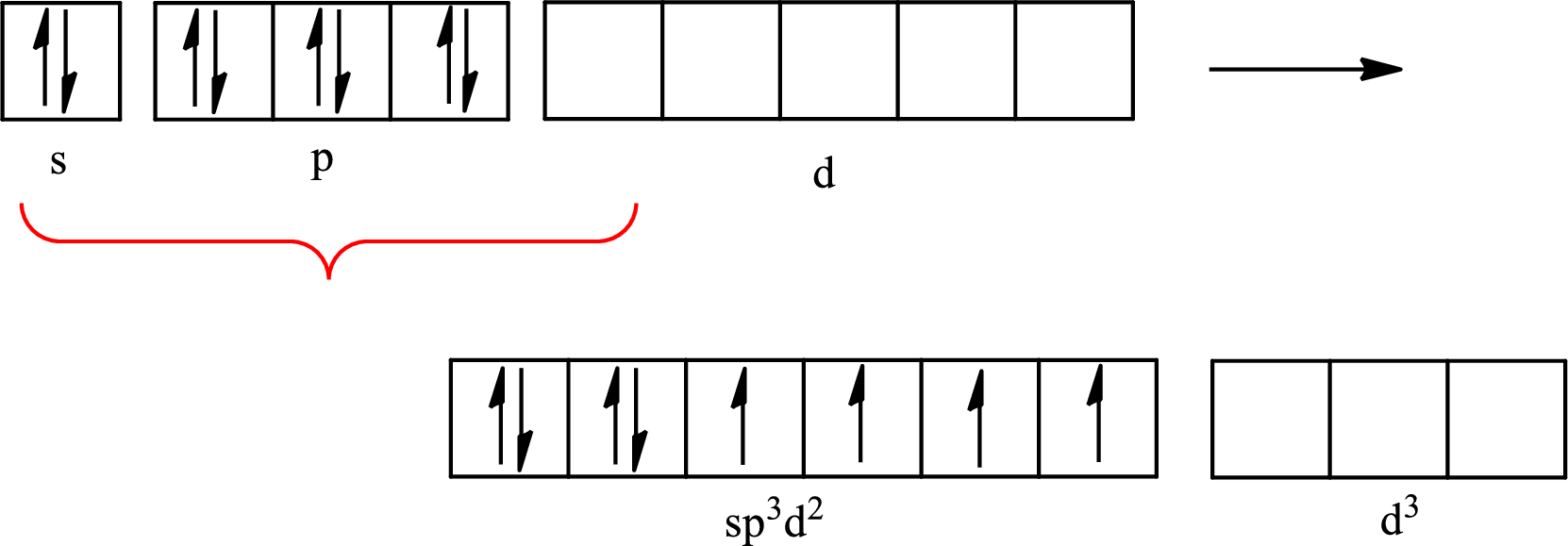
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom xenon in
(d)
Interpretation:
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom in
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
The partial orbital diagram is the one that shows the distribution of electrons in the valence shell only.
(d)
Answer to Problem 11.41P
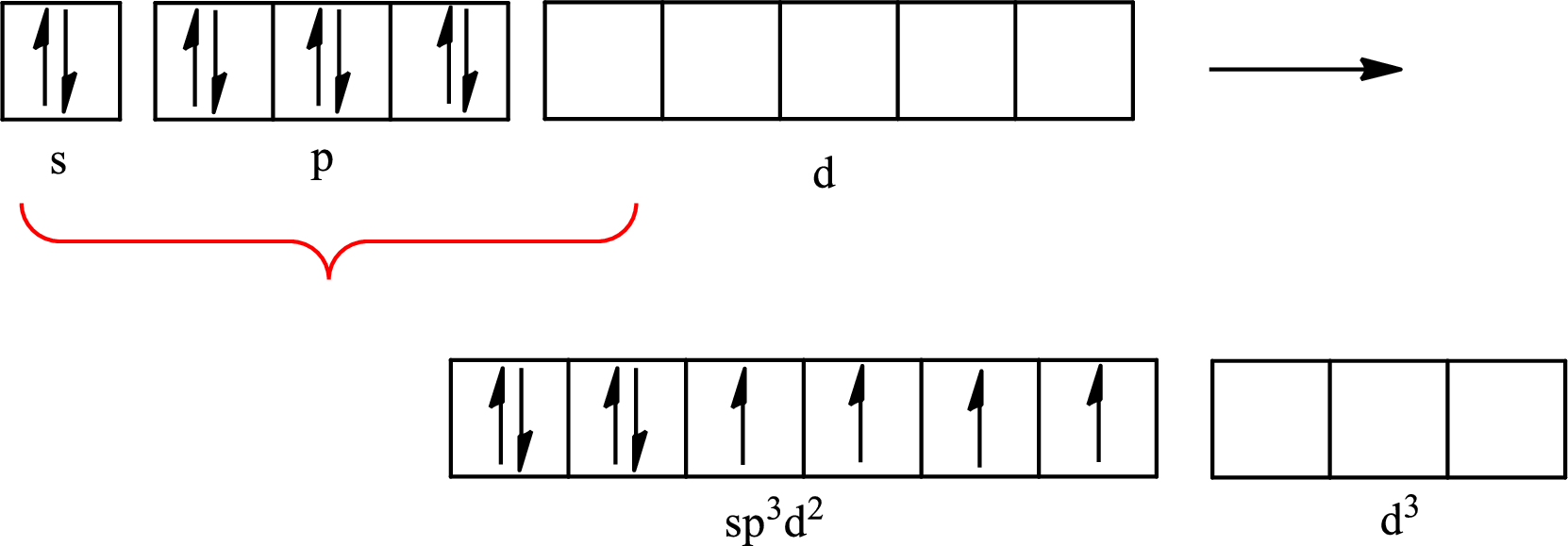
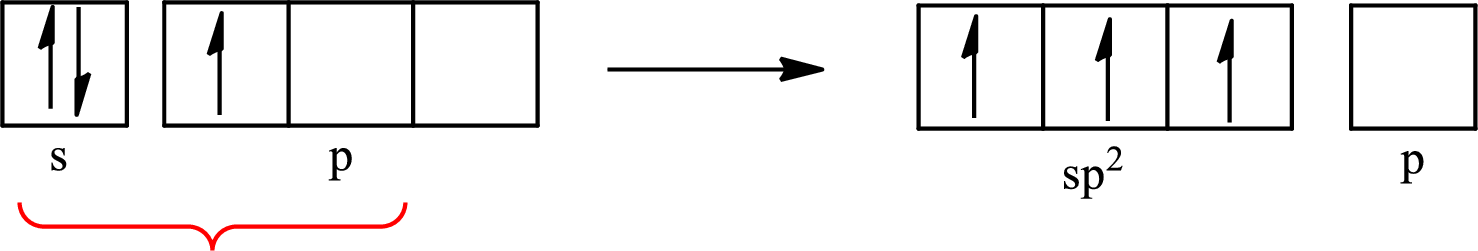
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom boron in
Explanation of Solution
The Lewis structure of
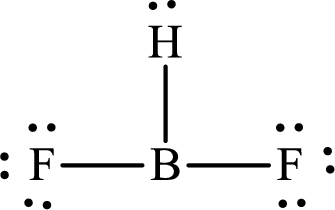
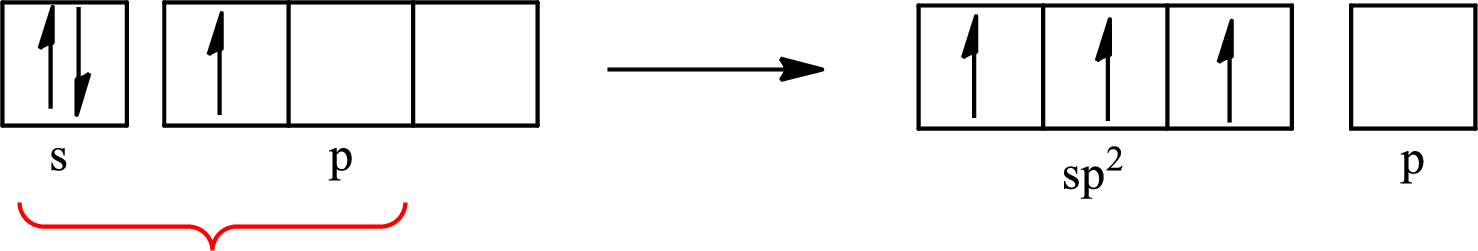
Boron forms one single bond with hydrogen and two single bonds with two fluorine atoms so three hybrid orbitals are required. The hybridization of boron in
The atomic number of boron is 5 so its electronic configuration is
The partial orbital diagram for an isolated
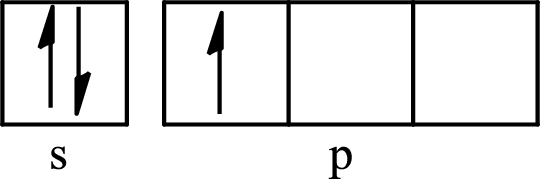
The partial orbital for hybridized
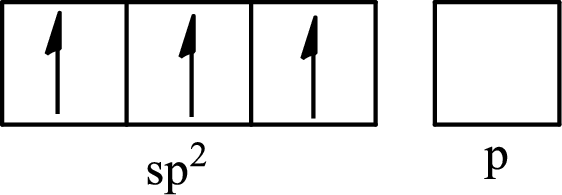
One s orbital and two p orbitals of central atom boron combine to form three
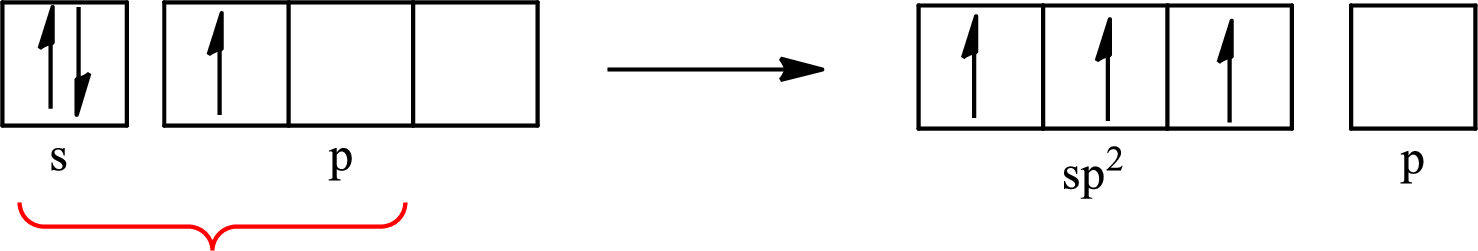
The partial orbital diagram that shows the formation of hybrid orbitals from the atomic orbitals of the central atom boron in
Want to see more full solutions like this?
Chapter 11 Solutions
CHEMISTRY:MOLECULAR NATURE...-ALEKS 360
- Draw the complete mechanism for the acid-catalyzed hydration of this alkene. esc 田 Explanation Check 1 888 Q A slock Add/Remove step Q F4 F5 F6 A བྲA F7 $ % 5 @ 4 2 3 & 6 87 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce W E R T Y U S D LL G H IK DD 요 F8 F9 F10 F1 * ( 8 9 0 O P J K L Z X C V B N M H He commandarrow_forwardExplanation Check F1 H₂O H₂ Pd 1) MCPBA 2) H3O+ 1) Hg(OAc)2, H₂O 2) NaBH4 OH CI OH OH OH hydration halohydrin formation addition halogenation hydrogenation inhalation hydrogenation hydration ☐ halohydrin formation addition halogenation formation chelation hydrogenation halohydrin formation substitution hydration halogenation addition Ohalohydrin formation subtraction halogenation addition hydrogenation hydration F2 80 F3 σ F4 F5 F6 1 ! 2 # 3 $ 4 % 05 Q W & Å © 2025 McGraw Hill LLC. All Rights Reserved. F7 F8 ( 6 7 8 9 LU E R T Y U A F9arrow_forwardShow the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forward
- Soap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forward
- Provide the mechanism for this transformation: *see imagearrow_forwardAssign all the signals individually (please assign the red, green and blue)arrow_forwardThe two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





