
Concept explainers
Predict the major products of the following reactions, including stereochemistry where appropriate.
- a. (R)-butan-2-ol+TsCI In pyridine
- b. (S)-2-butyl tosylate+NaBr
- c. cyclooctanol+NaOCI/HOAc
- d. cyclopentylmethanol+CrO3·pyridine·HCl
- e. cyclopentylmethanol+Na2Cr207/H2SO4
- f. cyclopentanol+HCl/ZnCl2
- g. n-butanol+HBr
- h. cyclooctylmethanol+CH3CH2MgBr
- i. potassium tert-butoxide+methyliodide
- j. sodium methoxide+tert-butyliodide
- k. cyclopentanol+H2SO4/heat
- l. product from (k)+OsO4/H2O2, then HIO4
- m. sodiumethoxide+1-bromobutane
- n. sodiumethoxide+2-methyl-2-bromobutane
- ○. octan-1-ol + DMSO + oxalyl chloride
- p. 4-cyclopentylhexan-1-ol + DMP reagent
(a)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Tosyl chloride in pyridine as a reagent is used to convert alcohols into respective tosylate esters through retention of configuration.
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between
The product of the given reaction is shown in Figure 1.
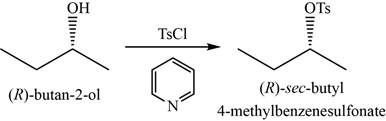
Figure 1
(b)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Sodium bromide is used to convert the compounds of tosylate ester into respective bromide through inversion of configuration.
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between
The product of the given reaction is shown in Figure 2.
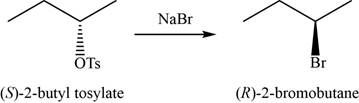
Figure 2
(c)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Sodium hypochlorite with acetic acid
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between cyclooctanol and
The product of the given reaction is shown in Figure 3.
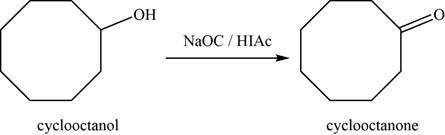
Figure 3
(d)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The Jones reagent
Answer to Problem 11.39SP
The major product of the given reaction is cyclopentanecarbaldehyde.
Explanation of Solution
The given reaction occurs between cyclopentylmethanol and
The product of the given reaction is shown in Figure 4.
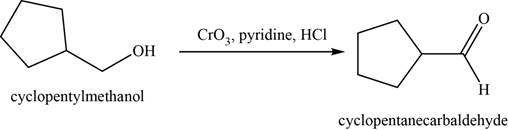
Figure 4
(e)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The Jones reagent
Answer to Problem 11.39SP
The major product of the given reaction is cyclopentanecarboxylic acid.
Explanation of Solution
The given reaction occurs between cyclopentylmethanol and
The product of the given reaction is shown in Figure 5.
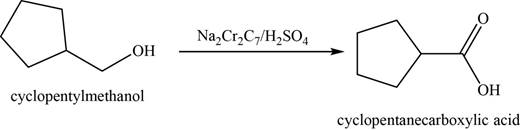
Figure 5
(f)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The Lucas reagent
Answer to Problem 11.39SP
The major product of the given reaction is chlorocyclopentane.
Explanation of Solution
The given reaction occurs between cyclopentanol and
The product of the given reaction is shown in Figure 6.
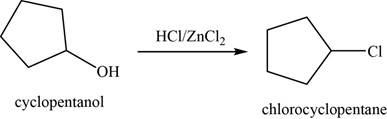
Figure 6
(g)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The halo acids like
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between
The product of the given reaction is shown in Figure 7.
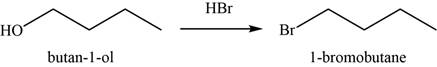
Figure 7
(h)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The reaction of alcohols with Grignard reagent leads to the formation of adduct complex of Grignard.
Answer to Problem 11.39SP
The major product of the given reaction is magnesium bromide cyclooctylmethanolate.
Explanation of Solution
The given reaction occurs between cyclooctylmethanol and
The product of the given reaction is shown in Figure 8.
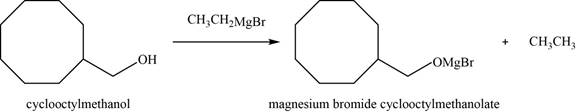
Figure 8
(i)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Methyl iodide is used to convert an alkoxide into ether with removal of halo-salts.
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between potassium tert-butoxide and methyliodide. Methyl iodide is used to convert an alkoxide into ether with removal of halogen salts.
The product of the given reaction is shown in Figure 9.
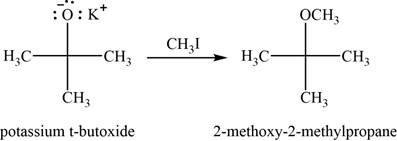
Figure 9
(j)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Sodium methoxide is a strong base, used for the conversion of bulkier haloalkane into an alkene with removal of an alcohol.
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between sodium methoxide and tert-butyliodide. Sodium methoxide is a strong base, used for the conversion of bulkier haloalkane into an alkene with the removal of an alcohol.
The product of the given reaction is shown in Figure 10.
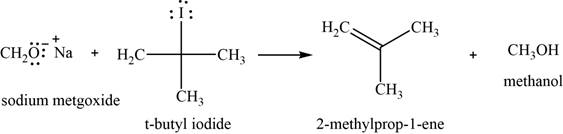
Figure 10
(k)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The sulphuric acid catalyzed reaction is used to convert alcohols into an alkene through dehydration and
Answer to Problem 11.39SP
The major product of the given reaction is cyclopentene.
Explanation of Solution
The given reaction occurs between cyclopentanol and
The sulphuric acid catalyzed reaction is used to convert alcohols into an alkene through dehydration and
The product of the given reaction is shown in Figure 11.
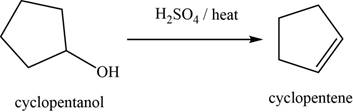
Figure 11
(l)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Osmium tetra oxide
Answer to Problem 11.39SP
The major product of the given reaction is glutaraldehyde.
Explanation of Solution
The given reaction occurs between cyclopentene (product from k) and
The product of the given reaction is shown in Figure 12.
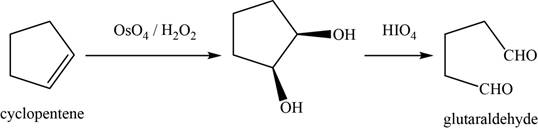
Figure 12
(m)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Sodium ethoxide is a strong base and used to convert haloalkanes into respective ether through
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between sodium ethoxide and
Sodium ethoxide is a strong base and used to convert haloalkanes into respective ether through
The product of the given reaction is shown in Figure 13.
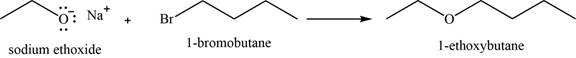
Figure 13
(n)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: Sodium ethoxide is a strong base and used to convert bulkier haloalkanes into respective alkenes.
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between sodium ethoxide and
Sodium ethoxide is a strong base and used to convert bulkier haloalkanes into respective alkenes.
The product of the given reaction is shown in Figure 14.
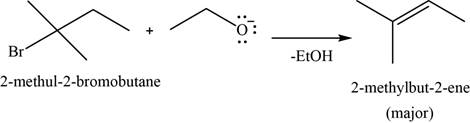
Figure 14
(o)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The Swern oxidation of alcohols to convert it into aldehydes are done by
Answer to Problem 11.39SP
The major product of the given reaction is octanal.
Explanation of Solution
The given reaction occurs between
The product of the given reaction is shown in Figure 15.
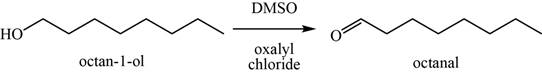
Figure 15
(p)
To determine: The major product of the given reaction, including stereochemistry if appropriate.
Interpretation: The major product of the given reaction, including stereochemistry if appropriate is to be predicted.
Concept introduction: The
Answer to Problem 11.39SP
The major product of the given reaction is
Explanation of Solution
The given reaction occurs between
The product of the given reaction is shown in Figure 16.
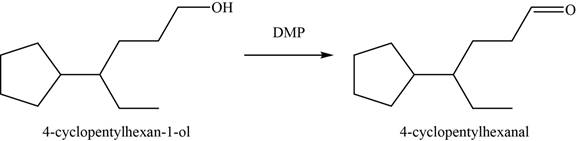
Figure 16
Want to see more full solutions like this?
Chapter 11 Solutions
ORGANIC CHEMISTRY
Additional Science Textbook Solutions
Fundamentals of Physics Extended
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
General, Organic, and Biological Chemistry - 4th edition
Human Anatomy & Physiology (2nd Edition)
Biology: Concepts and Investigations
- Indicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forwardFor the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.arrow_forward
- Name the following molecules using iupacarrow_forwardWrite the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophenarrow_forwardFor the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

