
(a)
Interpretation:
Line angle formula to be identified for 2,2,4-trimethylhexane.
Concept Introduction:
Example of
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 11.29P
The line angle formula for 2,2,4-trimethylhexane.
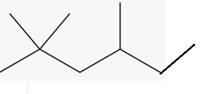
Explanation of Solution
The line angle formula for 2,2,4-trimethylhexane.
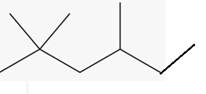
(b)
Interpretation:
Line angle formula to be identified for 2,2-dimethylpropane.
Concept Introduction:
Example of alkane are propane having formula C3 H8, butane having formula C4 H10 etc. Their molecular formula indicate the number of hydrogen is 2 more than twice the number of carbon in the structure and thus the derived formula for alkane is Cn H2n +2.
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 11.29P
The line angle formula for 2,2-dimethylpropane.

Explanation of Solution
The line angle formula for 2,2-dimethylpropane.

(c)
Interpretation:
Line angle formula to be identified for 3-ethyl-2,4,5-trimethyloctane.
Concept Introduction:
Example of alkane are propane having formula C3 H8, butane having formula C4 H10 etc. Their molecular formula indicate the number of hydrogen is 2 more than twice the number of carbon in the structure and thus the derived formula for alkane is Cn H2n +2.
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 11.29P
The line angle formula for 3-ethyl-2,4,5-trimethyloctane.
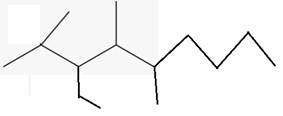
Explanation of Solution
The line angle formula for 3-ethyl-2,4,5-trimethyloctane.
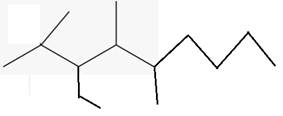
(d)
Interpretation:
Line angle formula to be identified for 5-butyl-2,2-dimethylnonane.
Concept Introduction:
Example of alkane are propane having formula C3 H8, butane having formula C4 H10 etc. Their molecular formula indicate the number of hydrogen is 2 more than twice the number of carbon in the structure and thus the derived formula for alkane is Cn H2n +2.
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 11.29P
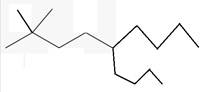
The line angle formula for 5-butyl-2,2-dimethylnonane.
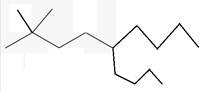
Explanation of Solution
The line angle formula for 5-butyl-2,2-dimethylnonane.
(e)
Interpretation:
Line angle formula to be identified for 4-isopropyloctane.
Concept Introduction:
Example of alkane are propane having formula C3 H8, butane having formula C4 H10 etc. Their molecular formula indicate the number of hydrogen is 2 more than twice the number of carbon in the structure and thus the derived formula for alkane is Cn H2n +2.
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 11.29P
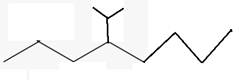
The line angle formula for 4-isopropyloctane.
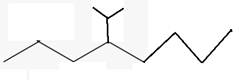
Explanation of Solution
The line angle formula for 4-isopropyloctane.
(f)
Interpretation:
Line angle formula to be identified for 3,3-dimethylpentane.
Concept Introduction:
Example of alkane are propane having formula C3 H8, butane having formula C4 H10 etc. Their molecular formula indicate the number of hydrogen is 2 more than twice the number of carbon in the structure and thus the derived formula for alkane is Cn H2n +2.
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 11.29P
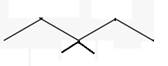
The line angle formula for 3,3-dimethylpentane.
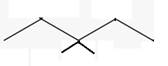
Explanation of Solution
The line angle formula for 3,3-dimethylpentane.
(g)
Interpretation:
Line angle formula to be identified for trans-1,3-dimethylcyclopentane.
Concept Introduction:
Example of alkane are propane having formula C3 H8, butane having formula C4 H10 etc. Their molecular formula indicate the number of hydrogen is 2 more than twice the number of carbon in the structure and thus the derived formula for alkane is Cn H2n +2.
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 11.29P
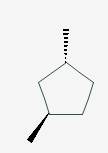
The line angle formula for trans-1,3-dimethylcyclopentane.
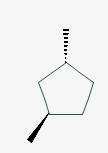
Explanation of Solution
The line angle formula for trans-1,3-dimethylcyclopentane.
(h)
Interpretation:
Line angle formula to be identified for Cis-1,2-diethylcyclobutane.
Concept Introduction:
Example of alkane are propane having formula C3 H8, butane having formula C4 H10 etc. Their molecular formula indicate the number of hydrogen is 2 more than twice the number of carbon in the structure and thus the derived formula for alkane is Cn H2n +2.
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 11.29P
The line angle formula for Cis-1,2-diethylcyclobutane.
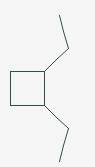
Explanation of Solution
The line angle formula for Cis-1,2-diethylcyclobutane.
Want to see more full solutions like this?
Chapter 11 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- elow are experimentally determined van Deemter plots of column efficiency, H, vs. flow rate. H is a quantitative measurement of band broadening. The left plot is for a liquid chromatography application and the night is for gas chromatography. Compare and contrast these two plots in terms of the three band broadening mechanisms presented in this activity. How are they similar? How do they differ? Justify your answers.? 0.4 H (mm) 0.2 0.1- 0.3- 0 0.5 H (mm) 8.0 7.0 6.0 5.0 4.0- 3.0 T +++ 1.0 1.5 0 2.0 4.0 Flow Rate, u (cm/s) 6.0 8.0 Flow Rate, u (cm/s)arrow_forwardPredict the products of this organic reaction: + H ZH NaBH3CN H+ n. ? Click and drag to start drawing a structure. Xarrow_forwardWhat is the missing reactant R in this organic reaction? + R H3O+ + • Draw the structure of R in the drawing area below. • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure.arrow_forward
- What would be the best choices for the missing reagents 1 and 3 in this synthesis? 1 1. PPh3 2. n-BuLi 2 • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure.arrow_forwardThe product on the right-hand side of this reaction can be prepared from two organic reactants, under the conditions shown above and below the arrow. Draw 1 and 2 below, in any arrangement you like. 1+2 NaBH₂CN H+ N Click and drag to start drawing a structure. X $arrow_forwardExplain what is the maximum absorbance of in which caffeine absorbs?arrow_forward
- Explain reasons as to why the amount of caffeine extracted from both a singular extraction (5ml Mountain Dew) and a multiple extraction (2 x 5.0ml Mountain Dew) were severely high when compared to coca-cola?arrow_forwardProtecting Groups and Carbonyls 6) The synthesis generates allethrolone that exhibits high insect toxicity but low mammalian toxicity. They are used in pet shampoo, human lice shampoo, and industrial sprays for insects and mosquitos. Propose detailed mechanistic steps to generate the allethrolone label the different types of reagents (Grignard, acid/base protonation, acid/base deprotonation, reduction, oxidation, witting, aldol condensation, Robinson annulation, etc.) III + VI HS HS H+ CH,CH,Li III I II IV CI + P(Ph)3 V ༼ Hint: no strong base added VI S VII IX HO VIII -MgBr HgCl2,HgO HO. isomerization aqeuous solution H,SO, ༽༽༤༽༽ X MeOH Hint: enhances selectivity for reaction at the S X ☑arrow_forwardDraw the complete mechanism for the acid-catalyzed hydration of this alkene. esc 田 Explanation Check 1 888 Q A slock Add/Remove step Q F4 F5 F6 A བྲA F7 $ % 5 @ 4 2 3 & 6 87 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce W E R T Y U S D LL G H IK DD 요 F8 F9 F10 F1 * ( 8 9 0 O P J K L Z X C V B N M H He commandarrow_forward
- Explanation Check F1 H₂O H₂ Pd 1) MCPBA 2) H3O+ 1) Hg(OAc)2, H₂O 2) NaBH4 OH CI OH OH OH hydration halohydrin formation addition halogenation hydrogenation inhalation hydrogenation hydration ☐ halohydrin formation addition halogenation formation chelation hydrogenation halohydrin formation substitution hydration halogenation addition Ohalohydrin formation subtraction halogenation addition hydrogenation hydration F2 80 F3 σ F4 F5 F6 1 ! 2 # 3 $ 4 % 05 Q W & Å © 2025 McGraw Hill LLC. All Rights Reserved. F7 F8 ( 6 7 8 9 LU E R T Y U A F9arrow_forwardShow the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forwardSoap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





