
Concept explainers
(a)
Interpretation:
The reaction and complete, detailed mechanism for the reaction is to be drawn.
Concept introduction:
A strong Bronsted acid such as
Answer to Problem 11.27P
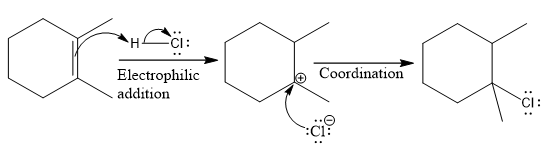
The overall reaction for the given compound is
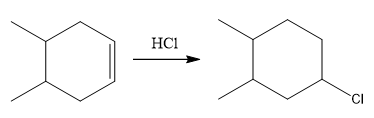
The complete mechanism for the above reaction is
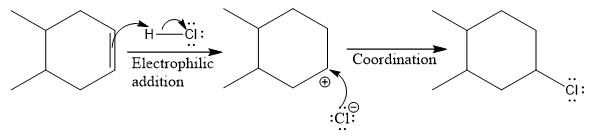
Explanation of Solution
The structure for the given compound
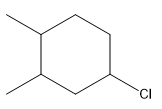
The compound shown above is the alkyl halide compound. This compound is formed by an electrophilic addition reaction of alkene with a strong Bronsted acid
The overall reaction for the given compound is

The above reaction completes in two steps. In the first step, the electrophilic addition step, the proton is added to one carbon with C=C bond, forming the carbocation intermediate. The second step is the coordination step in which the nucleophile attacks the carbocation to yield the product.
The complete mechanism for the above reaction is

The reaction and complete, detailed mechanism for the reaction is drawn on the basis of the mechanism of electrophilic addition reaction of alkenes with strong acid.
(b)
Interpretation:
The reaction and complete, detailed mechanism for the reaction is to be drawn.
Concept introduction:
Alkyl halide is the product of the electrophilic addition reaction of the alkene with a strong Bronsted acid such as
A strong Bronsted acid such as
Answer to Problem 11.27P
The overall reaction for the given compound is
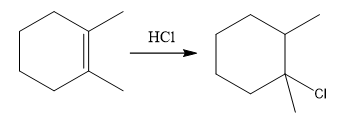
The complete mechanism for the above reaction is
Explanation of Solution
The structure for the given compound
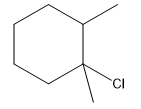
The compound shown above is the alkyl halide compound. This compound is formed by an electrophilic addition reaction of an alkene with a strong Bronsted acid
The overall reaction for the given compound is

The above reaction completes in two steps. In the first step, the electrophilic addition step, the proton is added to one carbon with C=C bond, forming the carbocation intermediate. The second step is the coordination step in which the nucleophile attacks the carbocation to yield the product.
The complete mechanism for the above reaction is

The reaction and complete, detailed mechanism for the reaction is drawn on the basis of the mechanism of electrophilic addition reaction of alkenes with strong acid.
(c)
Interpretation:
The reaction and complete, detailed mechanism for the reaction is to be drawn.
Concept introduction:
Alkyl halide is the product of the electrophilic addition reaction of the alkene with a strong Bronsted acid such as
A strong Bronsted acid such as
Answer to Problem 11.27P
The overall reaction for the given compound is
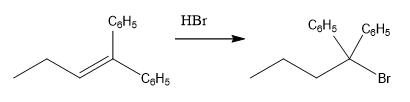
The complete mechanism for the above reaction is
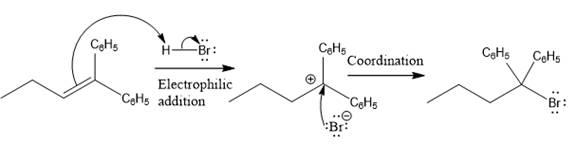
Explanation of Solution
The structure for the given compound
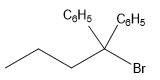
The compound shown above is the alkyl halide compound. This compound is formed by an electrophilic addition reaction of alkene with a strong Bronsted acid
The overall reaction for the given compound is

The above reaction completes in two steps. In the first step, the electrophilic addition step, the proton added to the internal carbon, forming the the more stable carbocation intermediate, which is tertiary and resonance stabilized. The second step is the coordination step in which the nucleophile attacks the carbocation to yield the product.
The complete mechanism for the above reaction is
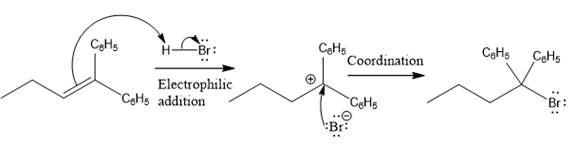
The reaction and complete, detailed mechanism for the reaction is drawn on the basis of the mechanism of electrophilic addition reaction of alkenes with strong acid.
(d)
Interpretation:
The reaction and complete, detailed mechanism for the reaction is to be drawn.
Concept introduction:
Alkyl halide is the product of the electrophilic addition reaction of the alkene with a strong Bronsted acid such as
A strong Bronsted acid such as
Answer to Problem 11.27P
The overall reaction for the given compound is
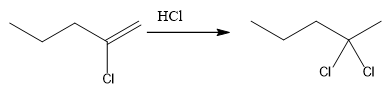
The complete mechanism for the above reaction is
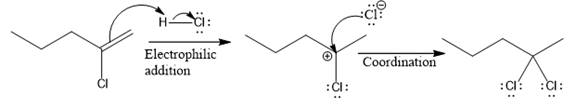
Explanation of Solution
The structure for the given compound
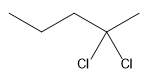
The compound shown above is the alkyl halide compound. This compound is formed by an electrophilic addition reaction of alkene with a strong Bronsted acid
The overall reaction for the given compound is

The above reaction completes in two steps. In the first step, the electrophilic addition step, the proton is added to the internal carbon, forming the the more stable carbocation intermediate, which is tertiary. The second step is the coordination step in which the nucleophile attacks the carbocation to yield the product.
The complete mechanism for the above reaction is
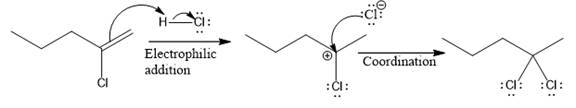
The reaction and complete, detailed mechanism for the reaction is drawn on the basis of the mechanism of electrophilic addition reaction of alkenes with strong acid.
Want to see more full solutions like this?
Chapter 11 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Provide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forwardGiven the attached data, provide the drawing for the corresponding structure.arrow_forward
