
Magnesium-28 decays by β emission to give aluminum-28. If yellow spheres represent
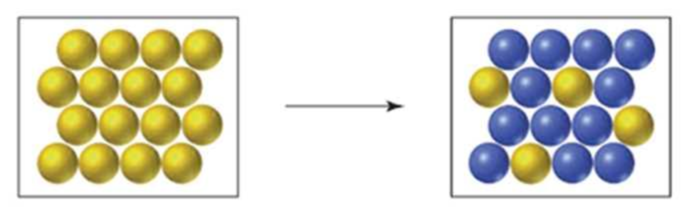
Interpretation:
The number of half-lives when
Concept Introduction:
Half-life:
The time required to reduce a radioactive isotope to half of its initial value.
Explanation of Solution
Figure 1
From the above figure,
We know that, yellow spheres are representing
The total number of yellow spheres present in first box is
The number of yellow spheres present in second box after beta decay is 4
So, the number of half-lives needed is
The number of half-lives when
Want to see more full solutions like this?
Chapter 11 Solutions
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
- Redox Chemistry: Give standard free energy changes expected for the following reactions:-Succinate -> fumarate (using FAD/FADH2)-Oxaloacetate -> Malate (using NAD/NADH)-NADH --> NAD+ (using FMN/FMNH2)-CoQ --> CoQH2 (using Cytochrome C)arrow_forwardGive examples of balanced redox reactions that match the following:-Catabolic-Anabolic-Oxidative-Reductivearrow_forwardIf there are 20uM of a GLUT2 transporter on the surface of a cell, each able to move 8 per second, and 50mM glucose outside of the cell, what is the flux into the cell in mM/sec?arrow_forward
- A transporter is responsible for antiporting calcium and glucose. The transporter brings glucose into the cell and sends calcium out of the cell. If blood [calcium] = 2.55mM and intracellular [calcium] = 7uM, blood [glucose] = 5.2mM, and intracellular [glucose] = 40uM, what is the free energy of transport? Assume a membrane potential of 62mV (negative inside).arrow_forwardAn ATP-coupled transporter is used to import 1 phosphate from the extracellular environment. Intracellular phosphate exists at 65mM, while it is 2mM outside.Assume a free energy change of ATP hydrolysis of -42.7 kJ/mol. What is the net free energy change of the coupled reaction? Assume a membrane potential of 70mV.arrow_forwardAnother transporter brings 3 chloride ions into the cell. Outside, chloride has a concentration of 107mM, and 4mM inside the cell. Assuming a membrane potential of 62mV (negative inside), what is the free energy of transport of these ions?arrow_forward
- For the Oxaloacetate -> Malate reaction, assume the normal ratio of NAD/NADH, what is the maximum ratio of Malate/Oxaloacetate that will allow reaction progress?arrow_forwardA particular particle is trying to cross a membrane by simple diffusion from a high concentration of 20mM to a low concentration of 20uM. If a membrane is 15uM in width, and the diffusion coefficient of the particle is 5 uM/sec, what is the influx in uM/sec?arrow_forwardMechanisms: 1. Give a full arrow-pushing mechanism for the hydrolysis of the gamma phosphate of ATP by an ATPase. 2. Give a full arrow pushing mechanism of the spontaneous redox reaction between NAD+/NADH and oxaloacetate/malate.arrow_forward
- Define the difference between primary and secondary active transport. Is one preferable to another?arrow_forwardWhich B vitamin is responsible for generating the following:- FAD/FMN-NAD/NADH/NADP/NADPH-Coenzyme Aarrow_forwardWhat is the free energy change of an NADH mediated reduction of acetaldehyde to ethanol if blood ethanol is 0.2% by volume, and [Acetaldehyde] = 9uM. Assume the ratio of NAD/NADH is the same as discussed in class.arrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning





