
(a)
Interpretation:
For the given reaction, a complete, detailed mechanism is to be drawn, and the major product, ignoring stereochemistry, is to be predicted.
Concept introduction:
An electrophilic addition reaction is an addition reaction where a
Answer to Problem 11.1P
The complete mechanism for the given reaction is
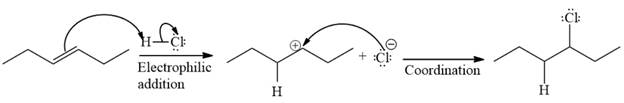
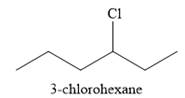
The major product of the reaction is
Explanation of Solution
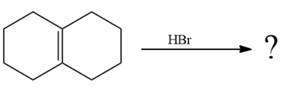
The given reaction is
![]()
The given reaction occurs in two steps. The electrophilic addition is the first elementary step. The
The product of an electrophilic addition reaction is formed as a result of the addition of the two parts of a Bronsted acid across a double bond, replacing one
(b)
Interpretation:
For the given reaction, the complete, detailed mechanism is to be drawn, and the major product is to be predicted.
Concept introduction:
An electrophilic addition reaction is an addition reaction where a
Answer to Problem 11.1P
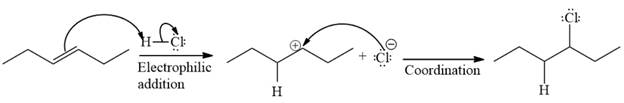
The complete mechanism of the given reaction is
The major product of the reaction is
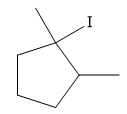
Explanation of Solution
The given reaction is
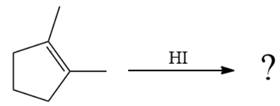
The given reaction occurs in two steps. The first elementary step is an electrophilic addition, where the
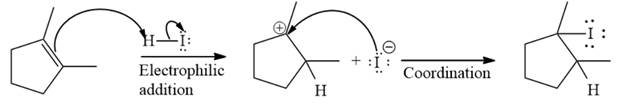
The product of an electrophilic addition reaction is formed as a result of the addition of the two parts of a Bronsted acid across a double bond, replacing one
(c)
Interpretation:
For the given reaction, the complete, detailed mechanism is to be drawn, and the major product is to be predicted.
Concept introduction:
An electrophilic addition reaction is an addition reaction where a
Answer to Problem 11.1P
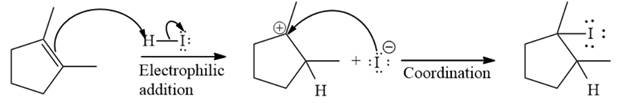
The complete mechanism of the given addition reaction is
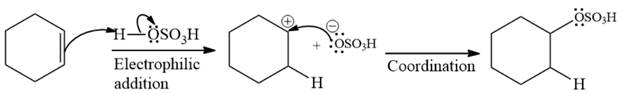
The major product of the reaction is
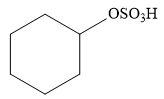
Explanation of Solution
The given addition reaction is
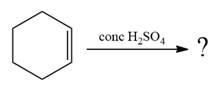
The given reaction occurs in two steps. The electrophilic addition is the first elementary step. The

The product of an electrophilic addition reaction is formed as a result of the addition of the two parts of a Bronsted acid across a double bond, replacing one
(d)
Interpretation:
For the given reaction, the complete, detailed mechanism is to be drawn, and the major product is to be predicted.
Concept introduction:
An electrophilic addition reaction is an addition reaction where a
Answer to Problem 11.1P
The complete mechanism of the given addition reaction is
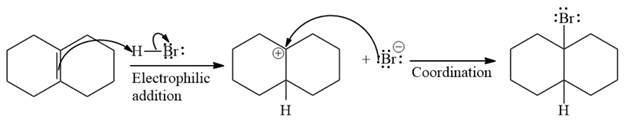
The major product of the reaction is
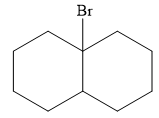
Explanation of Solution
The given addition reaction is
The given reaction occurs in two steps. The electrophilic addition is the first elementary step. The
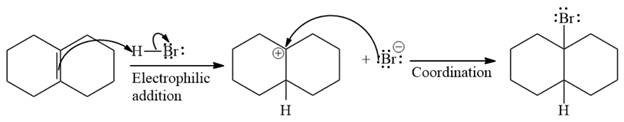
The product of an electrophilic addition reaction is formed as a result of the addition of the two parts of a Bronsted acid across a double bond, replacing one
Want to see more full solutions like this?
Chapter 11 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Nonearrow_forwardIn the phase diagram of steel (two components Fe and C), region A is the gamma austenite solid and region B contains the gamma solid and liquid. Indicate the degrees of freedom that the fields A and B have,arrow_forwardFor a condensed binary system in equilibrium at constant pressure, indicate the maximum number of phases that can exist.arrow_forward
- Part V. Label ad match the carbons in compounds Jane and Diane w/ the corresponding peak no. in the Spectra (Note: use the given peak no. To label the carbons, other peak no are intentionally omitted) 7 4 2 -0.13 -0.12 -0.11 -0.10 -0.08 8 CI Jane 1 -0.09 5 210 200 190 180 170 160 150 140 130 120 110 100 -8 90 f1 (ppm) 11 8 172.4 172.0 f1 (ppr HO CI NH Diane 7 3 11 80 80 -80 -R 70 60 60 2 5 -8 50 40 8. 170 160 150 140 130 120 110 100 90 -0 80 70 20 f1 (ppm) 15 30 -20 20 -60 60 -0.07 -0.06 -0.05 -0.04 -0.03 -0.02 -0.01 -0.00 -0.01 10 -0.17 16 15 56 16 -0.16 -0.15 -0.14 -0.13 -0.12 -0.11 -0.10 -0.09 -0.08 -0.07 -0.06 -0.05 -0.04 17.8 17.6 17.4 17.2 17.0 f1 (ppm) -0.03 -0.02 550 106 40 30 20 20 -0.01 -0.00 F-0.01 10 0arrow_forwardConsider the reaction of 2-methylpropane with a halogen. With which halogen will the product be almost exclusively 2-halo-2-methylpropane? 1. F2 2. Cl2 3. Br2 4. I2arrow_forwardNonearrow_forward
- Nonearrow_forwardn Feb 3 A T + 4. (2 pts) Draw the structure of the major component of the Limonene isolated. Explain how you confirmed the structure. 5. (2 pts) Draw the fragment corresponding to the base peak in the Mass spectrum of Limonene. 6. (1 pts) Predict the 1H NMR spectral data of R-Limonene. Proton NMR: 5.3 pon multiplet (H Ringarrow_forwardPart VI. Ca H 10 O is the molecular formula of compound Tom and gives the in the table below. Give a possible structure for compound Tom. 13C Signals summarized C1 C2 C3 C4 C5 C6 C7 13C shift (ppm) 23.5 27.0 33.0 35.8 127 162 205 DEPT-90 + DEPT-135 + +arrow_forward
- 2. Using the following data to calculate the value of AvapH o of water at 298K. AvapH o of water at 373K is 40.7 kJ/mol; molar heat capacity of liquid water at constant pressure is 75.2J mol-1 K-1 and molar heat capacity of water vapor at constant pressure is 33.6 J mol-1 K-1.arrow_forwardPart VII. Below are the 'HNMR 13 3 C-NMR, COSY 2D- NMR, and HSQC 20-NMR (Similar with HETCOR but axes are reversed) spectra of an organic compound with molecular formula C6H13 O. Assign chemical shift values to the H and c atoms of the compound. Find the structure. Show complete solutions. Predicted 1H NMR Spectrum ли 4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 f1 (ppm)arrow_forward3. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-pentene. expanded structure: Condensed structure: Skeletal formula: 4. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-methyl-3-heptene. expanded structure: Condensed structure: Skeletal formula: following structurearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
