
(a)
Interpretation:
The hybridization of the central atom that corresponds to a trigonal planar arrangement is to be determined.
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
(a)
Answer to Problem 11.1P
The hybridization of the central atom in trigonal planar is
Explanation of Solution
The molecule that has trigonal planar geometry is
The partial orbital diagram of an isolated
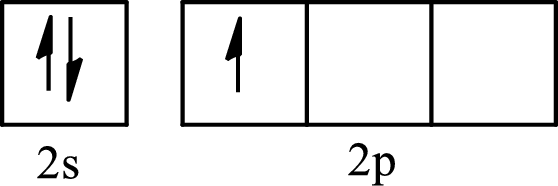
The partial orbital diagram of a hybridized
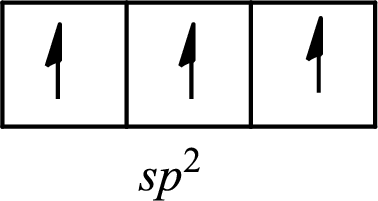
The half-filled
The hybridization of the central atom in trigonal planar is
(b)
Interpretation:
The hybridization of the central atom that corresponds to the octahedral arrangement is to be determined.
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
(b)
Answer to Problem 11.1P
The hybridization of the central atom in octahedral is
Explanation of Solution
The molecule that has octahedral geometry is
The partial orbital diagram of an isolated
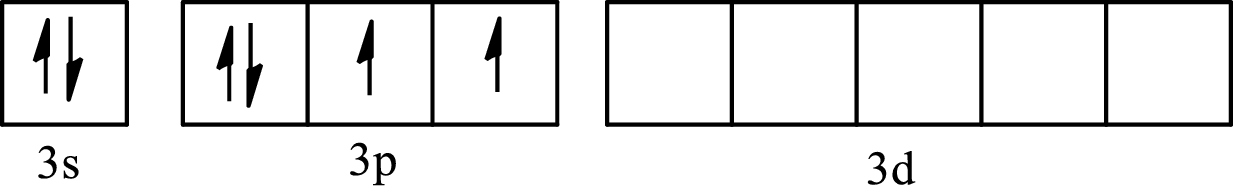
The partial orbital diagram of a hybridized
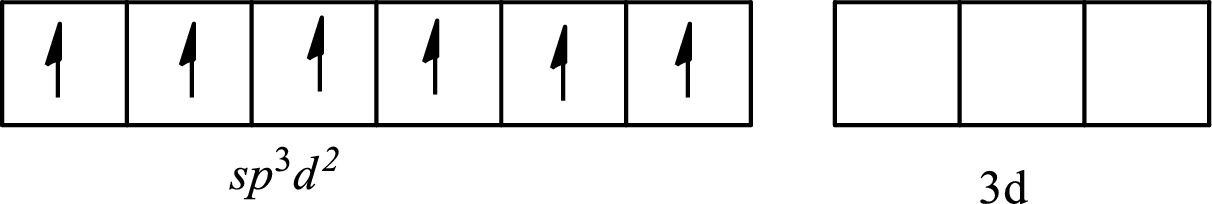
The six half-filled
The hybridization of the central atom in octahedral is
(c)
Interpretation:
The hybridization of the central atom that corresponds to a linear arrangement is to be determined.
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
(c)
Answer to Problem 11.1P
The hybridization of the central atom in a linear arrangement is
Explanation of Solution
The molecule that has a linear arrangement is
The partial orbital diagram of an isolated
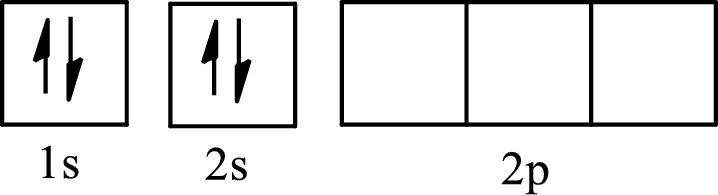
The partial orbital diagram of a hybridized
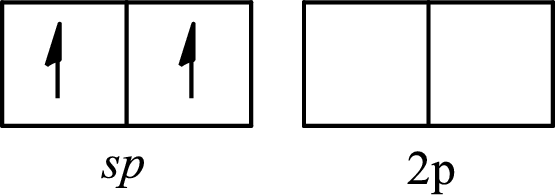
The two half-filled
The hybridization of the central atom in a linear arrangement is
(d)
Interpretation:
The hybridization of the central atom that corresponds to a tetrahedral arrangement is to be determined.
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
(d)
Answer to Problem 11.1P
The hybridization of the central atom in a tetrahedral arrangement is
Explanation of Solution
The molecule that has a tetrahedral arrangement is
The partial orbital diagram of an isolated
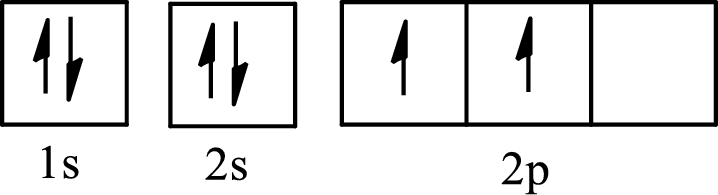
The partial orbital diagram of a hybridized
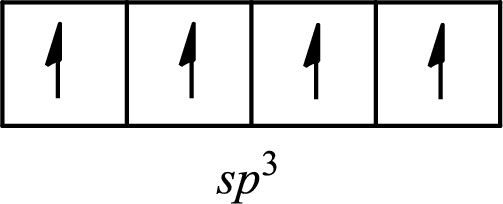
The four half-filled
The hybridization of the central atom in a tetrahedral arrangement is
(e)
Interpretation:
The hybridization of the central atom that corresponds to a trigonal bipyramidal arrangement is to be determined.
Concept introduction:
The atomic orbital is the wave function that is used to find the probability to find an electron around the nucleus of an atom. It is the space around the nucleus of an atom where the electrons are supposed to be found.
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital.
(e)
Answer to Problem 11.1P
The hybridization of the central atom in a trigonal bipyramidal arrangement is
Explanation of Solution
The molecule that has a trigonal bipyramidal arrangement is
The partial orbital diagram of an isolated
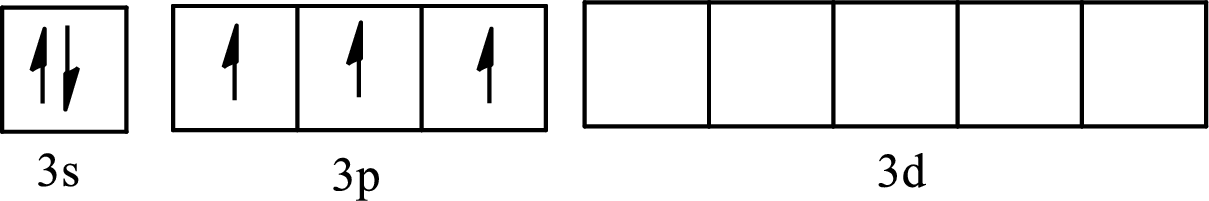
The partial orbital diagram of a hybridized
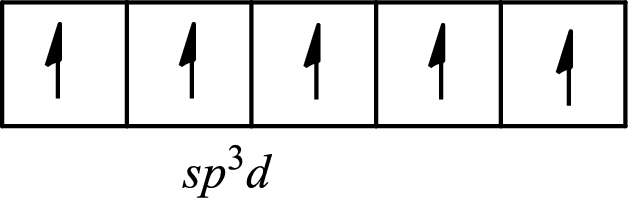
The five half-filled
The hybridization of the central atom in a trigonal bipyramidal arrangement is
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry: The Molecular Nature of Matter and Change
- Consider the following nucleophilic substitution reaction. The compound listed above the arrow is the solvent for the reaction. If nothing is listed over the arrow, then the nucleophile is also the solvent for the reaction. Part 1 of 2 Br CH,CN + I¯ What is the correct mechanism for the reaction? Select the single best answer. @SN2 ○ SN 1 Part: 1/2 Part 2 of 2 Draw the products for the reaction. Include both the major organic product and the inorganic product. If more than one stereoisomer is possible, draw only one stereoisomer. Include stereochemistry where relevant. Click and drag to start drawing a structure. X હૈarrow_forward20.33 Think-Pair-Share (a) Rank the following dienes and dienophiles in order of increasing reactivity in the Diels-Alder reaction. (i) CO₂Et (ii) COEt || CO₂Et MeO MeO (b) Draw the product that results from the most reactive diene and most reactive dienophile shown in part (a). (c) Draw a depiction of the orbital overlap involved in the pericyclic reaction that oc- curs between the diene and dienophile in part (b). (d) Is the major product formed in part (b) the endo or exo configuration? Explain your reasoning.arrow_forward20.40 The following compound undergoes an intramolecular Diels-Alder reaction to give a tricyclic product. Propose a structural formula for the product. CN heat An intramolecular Diels-Alder adductarrow_forward
- What is the reaction mechanism for this?arrow_forwardWhat is the reaction mechanism for this?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. + Drawing Arrows CH3ONA, CH3OH heat : Br:O Na → H H Br Na + H H H H H :0: .H + Undo Reset Done Q CH3 Drag To Pan +arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





