
Concept explainers
The relative rate of radical bromination is 1;82;1640 for 1°;2°;3° hydrogens, respectively. Draw all of the monobrominated products that you might obtain from the radical bromination of the compounds below. Calculate the relative percentage of each.
(a) methylcyclobutane
(b) 3,3-dimethylpentane
(c) 3-methylpentane
a) Methylcyclobutane
Interpretation:
The relative rate of radical bromination is 1:82:1640 for 1°, 2° and 3° hydrogens respectively. The structures of all the monobrominated products obtainable from the radical bromination of methylcyclobutane are to be drawn. The relative percentage of their formation is also to be calculated.
Concept introduction:
During radical bromination all types of hydrogens present in a compound are replaced by bromine to yield different products. The number of each type of hydrogen present in the compound and their relative rates of bromination are calculated seperately. The relative percentage of formation of a particular type of hydrogen can be calculated from the total rate of bromination of all types of hydrogens and that of the particular hydrogen.
To draw:
The structures of all the monobrominated products obtainable from the radical bromination of methylcyclobutane.
To calculate:
The relative percentage of formation of each monobromination product.
Answer to Problem 48AP
Four different monochlorination products are possible by the radical bromination of methylcyclohexane. They are bromomethylcyclobutane(I), 1-bromo-1-methylcyclobutane(II), 1-bromo-2-methylcyclobutane(III) and 1-bromo-3-methylcyclobutane(IV).
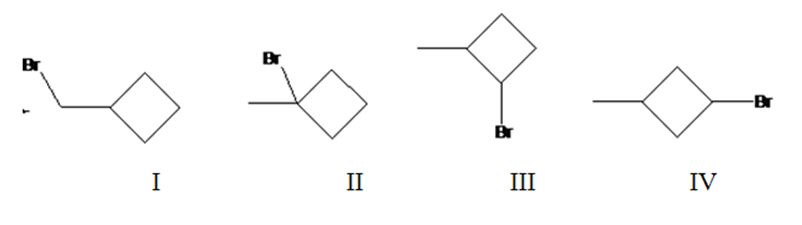
The relative percentage of 1°, 2° and 3° hydrogens in methylcyclobutane is 0.15: 23.1: 76.8.
Explanation of Solution
Methylcyclobutane has four types of hydrogens, one in methyl, a second on C1 to which methyl group is attached, a third one on C2 and C4 and a fourth one on C3. It has three 1° hydrogens, six 2° hydrogens and one 3° hydrogen. Hence four monochlorination products are possible.
The relative rate of radical bromonation of 1° hydrogens = 3 x 1 = 3.
The relative rate of radical bromonation of 2° hydrogens = 6 x 82 = 492.
The relative rate of radical bromonation of 3° hydrogens = 1 x 1640 = 1640.
Total rate of radical bromonation of all hydrogens = 3+492+1640 = 2135.
Therefore the relative percentage of
Radical bromonation of 1° hydrogens = 3/2135 x 100= 0.15%.
Radical bromonation of 2° hydrogens = 492/2135 x 100= 23.1%.
Radical bromonation of 3° hydrogens = 1640/2135 x 100= 76.8%.
Four different monochlorination products are possible by the radical bromination of methylcyclohexane. They are bromomethylcyclobutane(I), 1-bromo-1-methylcyclobutane(II), 1-bromo-2-methylcyclobutane(III) and 1-bromo-3-methylcyclobutane(IV).

The relative percentage of 1°, 2° and 3° hydrogens in methylcyclobutane is 0.15: 23.1: 76.8.
b) 3,3-dimethylpentane
Interpretation:
The relative rate of radical bromination is 1:82:1640 for 1°, 2° and 3° hydrogens respectively. The structures of all the monobrominated products obtainable from the radical bromination of 3,3-dimethylpentane are to be drawn. The relative percentage of their formation is also to be calculated.
Concept introduction:
During radical bromination all types of hydrogens present in a compound are replaced by bromine to yield different products. The number of each type of hydrogen present in the compound and their relative rates of bromination are calculated seperately. The relative percentage of formation of a particular type of hydrogen can be calculated from the total rate of bromination of all types of hydrogens and that of the particular hydrogen.
To draw:
The structures of all the monobrominated products obtainable from the radical bromination of 3,3-dimethylpentane.
To calculate:
The relative percentage of formation of each monobromination product.
Answer to Problem 48AP
Three different monochlorination products are possible by the radical bromination of 3-bromomethyl-3-methylpentane. They are bromomethylcyclobutane(I), 2-bromo-3,3-dimethylpentane(II) and 1-bromo-3,3-dimethylpentane (III).
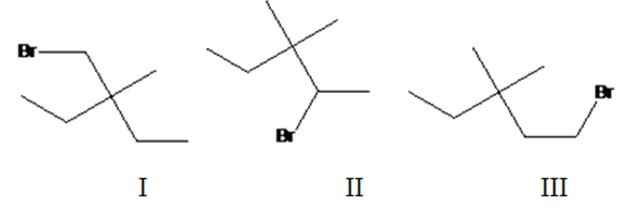
The relative percentage of 10and 20 hydrogens in 3,3-dimethylpentane is 3.6:96.4.
Explanation of Solution
3,3-Dimethylpentane has two types of hydrogens, one in methyl groups and other in CH2 attached to methyl group. Hence two monochlorination products are possible. It has twelve 1° hydrogens and four 2° hydrogen atoms.
The relative rate of radical bromonation of 1° hydrogens = 12 x 1 = 12.
The relative rate of radical bromonation of 2° hydrogens = 4 x 82 = 328.
Total rate of radical bromonation of all hydrogens = 12+328 = 340.
Therefore the relative percentage of
Rdical bromonation of 1° hydrogens = 12/340 x 100= 3.6%.
Rdical bromonation of 2° hydrogens = 328/340 x 100=96.4%.
Three different monochlorination products are possible by the radical bromination of 3-bromomethyl-3-methylpentane. They are bromomethylcyclobutane(I), 2-bromo-3,3-dimethylpentane(II) and 1-bromo-3,3-dimethylpentane (III).

The relative percentage of 1° and 2° hydrogens in 3,3-dimethylpentane is 3.6:96.4.
c) 3-methylpentane
Interpretation:
The relative rate of radical bromination is 1:82:1640 for 1°, 2° and 3° hydrogens respectively. The structures of all the monobrominated products obtainable from the radical bromination of 3-methylpentane are to be drawn. The relative percentage of their formation is also to be calculated.
Concept introduction:
During radical bromination all types of hydrogens present in a compound are replaced by bromine to yield different products. The number of each type of hydrogen present in the compound and their relative rates of bromination are calculated seperately. The relative percentage of formation of a particular type of hydrogen can be calculated from the total rate of bromination of all types of hydrogens and that of the particular hydrogen.
To draw:
The structures of all the monobrominated products obtainable from the radical bromination of 3-methylpentane.
To calculate:
The relative percentage of formation of each monobromination product.
Answer to Problem 48AP
Four different monochlorination products are possible by the radical bromination of 3-methylpentane. They are bromomethylpentane(I), 3-bromo-3-methylpentane(II), 2-bromo-3-methylpentane(III) and 1-bromo-3-methylpentane(IV).
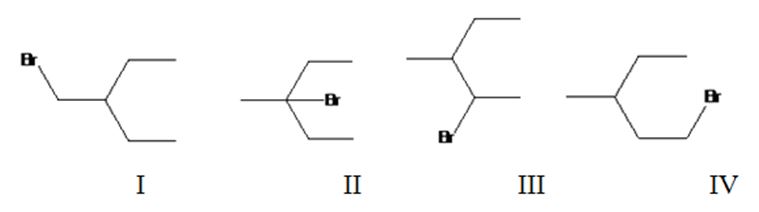
The relative percentage of 1°, 2° and 3° hydrogens in 3,3-dimethylpentane is 0.46:16.6:82.9.
Explanation of Solution
3,3-Dimethylpentane has four types of hydrogens, one in methyl at C1, another methyl groups attached to CH2, a third in C2 and a fourth in CH2. It has nine 1° hydrogens, four 2° hydrogens and one 3° hydrogen. Hence four monochlorination products are possible.
The relative rate of radical bromonation of 1° hydrogens = 9 x 1 = 9.
The relative rate of radical bromonation of 2° hydrogens = 4 x 82 = 328.
The relative rate of radical bromonation of 3° hydrogens = 1 x 1640 = 1640.
Total rate of radical bromonation of all hydrogens = 9+328+1640 = 1977.
Therefore the relative percentage of
Radical bromonation of 1° hydrogens = 9/1977x100= 0.46%.
Radical bromonation of 2° hydrogens = 328/1977x100= 16.6%.
Radical bromonation of 3° hydrogens = 1640/1937x100= 82.9%.
Four different monochlorination products are possible by the radical bromination of 3-methylpentane. They are bromomethylpentane(I), 3-bromo-3-methylpentane(II), 2-bromo-3-methylpentane(III) and 1-bromo-3-methylpentane(IV).

The relative percentage of 1°, 2° and 3° hydrogens in 3,3-dimethylpentane is 0.46:16.6:82.9.
Want to see more full solutions like this?
Chapter 10 Solutions
EBK ORGANIC CHEMISTRY
- Explain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forward
- Using Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forwardThe molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
