
Concept explainers
a)
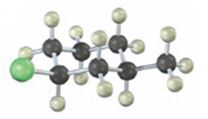
Interpretation:
The IUPAC name of the
Concept introduction:
The longest continuous carbon chain in the molecule is chosen. The chain is numbered from the end which gives lowest number to the substituent either halo or alkyl group present. If different halogens are present they are numbered and listed in the alphabetical order while writing the name. If same two alternatives exist for different substituents, then the chain is numbered from the end that gives lowest number to the substituent that has alphabetical preference.
To give:
The IUPAC name of the alkyl halide shown.
Answer to Problem 14VC
The alkyl halide given is
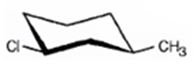
Its IUPAC name is cis- 1- chloro-3-methylcyclohexane.
Explanation of Solution
The compound has a cyclohexane ring with a Cl atom on C1 and a methyl on C3 both at equatorial positions. Hence its name is cis- 1- chloro-3-methylcyclohexane.
The IUPAC name of the alkyl halide shown is cis- 1- chloro-3-methylcyclohexane.
b)
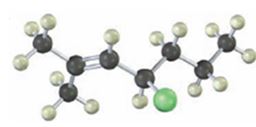
Interpretation:
The IUPAC name of the alkyl halide is to be given.
Concept introduction:
The longest continuous carbon chain in the molecule is chosen. The chain is numbered from the end which gives lowest number to the substituent either halo or alkyl group present. If different halogens are present they are numbered and listed in the alphabetical order while writing the name. If same two alternatives exist for different substituents, then the chain is numbered from the end that gives lowest number to the substituent that has alphabetical preference.
To give:
The IUPAC name of the alkyl halide shown.
Answer to Problem 14VC
The alkyl halide given is
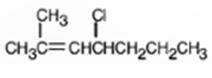
Its IUPAC name is 4-chloro-2-methyl-2-heptene.
Explanation of Solution
The compound has a seven carbon straight chain with a double bond between C2 & C3, a Cl atom on C4 and a methyl on C3. Hence its name is 4-chloro-2-methyl-2-heptene.
The IUPAC name of the alkyl halide shown is 4-chloro-2-methyl-2-heptene.
Want to see more full solutions like this?
Chapter 10 Solutions
Bundle: Organic Chemistry, 9th, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
- 1. Answer the questions about the following reaction: (a) Draw in the arrows that can be used make this reaction occur and draw in the product of substitution in this reaction. Be sure to include any relevant stereochemistry in the product structure. + SK F Br + (b) In which solvent would this reaction proceed the fastest (Circle one) Methanol Acetone (c) Imagine that you are working for a chemical company and it was your job to perform a similar reaction to the one above, with the exception of the S atom in this reaction being replaced by an O atom. During the reaction, you observe the formation of three separate molecules instead of the single molecule obtained above. What is the likeliest other products that are formed? Draw them in the box provided.arrow_forward3. For the reactions below, draw the arrows corresponding to the transformations and draw in the boxes the reactants or products as indicated. Note: Part A should have arrows drawn going from the reactants to the middle structure and the arrows on the middle structure that would yield the final structure. For part B, you will need to draw in the reactant before being able to draw the arrows corresponding to product formation. A. B. Rearrangement ΘΗarrow_forward2. Draw the arrows required to make the following reactions occur. Please ensure your arrows point from exactly where you want to exactly where you want. If it is unclear from where arrows start or where they end, only partial credit will be given. Note: You may need to draw in lone pairs before drawing the arrows. A. B. H-Br 人 C Θ CI H Cl Θ + Br Oarrow_forward
- 4. For the reactions below, draw the expected product. Be sure to indicate relevant stereochemistry or formal charges in the product structure. a) CI, H e b) H lux ligh Br 'Harrow_forwardArrange the solutions in order of increasing acidity. (Note that K (HF) = 6.8 x 10 and K (NH3) = 1.8 × 10-5) Rank solutions from least acidity to greatest acidity. To rank items as equivalent, overlap them. ▸ View Available Hint(s) Least acidity NH&F NaBr NaOH NH,Br NaCIO Reset Greatest acidityarrow_forward1. Consider the following molecular-level diagrams of a titration. O-HA molecule -Aion °° о ° (a) о (b) (c) (d) a. Which diagram best illustrates the microscopic representation for the EQUIVALENCE POINT in a titration of a weak acid (HA) with sodium. hydroxide? (e)arrow_forward
- Answers to the remaining 6 questions will be hand-drawn on paper and submitted as a single file upload below: Review of this week's reaction: H₂NCN (cyanamide) + CH3NHCH2COOH (sarcosine) + NaCl, NH4OH, H₂O ---> H₂NC(=NH)N(CH3)CH2COOH (creatine) Q7. Draw by hand the reaction of creatine synthesis listed above using line structures without showing the Cs and some of the Hs, but include the lone pairs of electrons wherever they apply. (4 pts) Q8. Considering the Zwitterion form of an amino acid, draw the Zwitterion form of Creatine. (2 pts) Q9. Explain with drawing why the C-N bond shown in creatine structure below can or cannot rotate. (3 pts) NH2(C=NH)-N(CH)CH2COOH This bond Q10. Draw two tautomers of creatine using line structures. (Note: this question is valid because problem Q9 is valid). (4 pts) Q11. Mechanism. After seeing and understanding the mechanism of creatine synthesis, students should be ready to understand the first half of one of the Grignard reactions presented in a past…arrow_forwardPropose a synthesis pathway for the following transformations. b) c) d)arrow_forwardThe rate coefficient of the gas-phase reaction 2 NO2 + O3 → N2O5 + O2 is 2.0x104 mol–1 dm3 s–1 at 300 K. Indicate whether the order of the reaction is 0, 1, or 2.arrow_forward
- 8. Draw all the resonance forms for each of the following molecules or ions, and indicate the major contributor in each case, or if they are equivalent. (4.5 pts) (a) PH2 سمةarrow_forward3. Assign absolute configuration (Rors) to each chirality center. a. H Nitz C. он b. 0 H-C. C H 7 C. ་-4 917-417 refs H 1つ ८ ડુ d. Но f. -2- 01 Ho -OH 2HNarrow_forwardHow many signals do you expect in the H NMR spectrum for this molecule? Br Br Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: In this question, any multiplet is counted as one signal. Number of signals in the 'H NMR spectrum. For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional Hs to color in top molecule For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional Hs to color in bottom moleculearrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

