
Organic Chemistry, Books a la Carte Edition (8th Edition)
8th Edition
ISBN: 9780134074580
Author: Bruice, Paula Yurkanis
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 10, Problem 94P
Interpretation Introduction
Interpretation:
The product should be given, when heating of vicinal
Concept introduction:
The pinacol–pinacolone rearrangement:
Pinacol–pinacolone rearrangement is a conversion of 1,2-diol to a carbonyl compound in the presence of acid via 1,2- shift is called rearrangement is called Pinacol–pinacolone rearrangement.
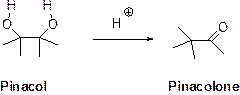
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
An unknown weak acid with a concentration of 0.410 M has a pH of 5.600. What is the Ka of the weak acid?
(racemic)
19.84 Using your reaction roadmaps as a guide, show how to convert 2-oxepanone and ethanol
into 1-cyclopentenecarbaldehyde. You must use 2-oxepanone as the source of all carbon
atoms in the target molecule. Show all reagents and all molecules synthesized along
the way.
&
+ EtOH
H
2-Oxepanone
1-Cyclopentenecarbaldehyde
R₂
R₁
R₁
a
R
Rg
Nu
R₂
Rg
R₁
R
R₁₂
R3
R
R
Nu enolate forming
R₁ R
B-Alkylated carbonyl
species or amines
Cyclic B-Ketoester
R₁₁
HOB
R
R₁B
R
R₁₂
B-Hydroxy carbonyl
R
diester
R2 R3
R₁
RB
OR
R₂ 0
aB-Unsaturated carbonyl
NaOR
Aldol
HOR
reaction
1) LDA
2) R-X
3) H₂O/H₂O
ketone,
aldehyde
1) 2°-amine
2) acid chloride
3) H₂O'/H₂O
0
O
R₁
R₁
R
R₁
R₁₂
Alkylated a-carbon
R₁
H.C
R₁
H.C
Alkylated methyl ketone
acetoacetic
ester
B-Ketoester
ester
R₁
HO
R₂ R
B-Dicarbonyl
HO
Alkylated carboxylic acid
malonic ester
Write the reagents required to bring about each reaction next to the arrows shown.
Next, record any regiochemistry or stereochemistry considerations relevant to the
reaction. You should also record any key aspects of the mechanism, such as forma-
tion of an important intermediate, as a helpful reminder. You may want to keep
track of all reactions that make carbon-carbon bonds, because these help you build
large molecules from smaller fragments. This especially applies to the reactions in…
Chapter 10 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
Ch. 10.1 - Why are NH3 and CH3NH2 no longer nucleophiles when...Ch. 10.1 - Explain the difference in reactivity between...Ch. 10.1 - Prob. 5PCh. 10.1 - Prob. 6PCh. 10.1 - Prob. 8PCh. 10.2 - Prob. 9PCh. 10.3 - Prob. 11PCh. 10.3 - Show how 1-propanol can be converted into the...Ch. 10.4 - Which of the following alcohols dehydrates the...Ch. 10.4 - Prob. 14P
Ch. 10.4 - Prob. 15PCh. 10.4 - Propose a mechanism for each of the following...Ch. 10.4 - Draw the product of each of the following...Ch. 10.4 - Explain why the following alcohols, when heated...Ch. 10.4 - What stereoisomers are formed in the following...Ch. 10.4 - Prob. 20PCh. 10.4 - What alcohol would you treat with phosphorus...Ch. 10.5 - Prob. 22PCh. 10.6 - What are the major products obtained when each of...Ch. 10.6 - Prob. 26PCh. 10.7 - Prob. 27PCh. 10.7 - Would you expect the reactivity of a five-membered...Ch. 10.7 - Prob. 29PCh. 10.7 - What products are obtained from the reaction of...Ch. 10.7 - Prob. 31PCh. 10.7 - Prob. 32PCh. 10.7 - Prob. 33PCh. 10.8 - Draw the mechanism for formation of the two...Ch. 10.8 - Prob. 35PCh. 10.8 - Prob. 36PCh. 10.8 - How do the major products obtained from...Ch. 10.8 - Explain why the two arene oxides in Problem 38...Ch. 10.8 - Which compound is more likely to be carcinogenic?Ch. 10.8 - Three arene oxides can be obtained from...Ch. 10.9 - Explain why the half-life (the time it takes for...Ch. 10.10 - Prob. 43PCh. 10.10 - Prob. 44PCh. 10.10 - Prob. 45PCh. 10.10 - Prob. 46PCh. 10.10 - Prob. 47PCh. 10.10 - Describe a synthesis for each of the following...Ch. 10.11 - Using an alkyl halide and a thiol as starting...Ch. 10.11 - The following three nitrogen mustards were studied...Ch. 10.11 - Why is melphalan a good cancer drug?Ch. 10.11 - Prob. 53PCh. 10.12 - Propose a mechanism for the following reaction:Ch. 10 - Prob. 55PCh. 10 - Which compound is more likely to be carcinogenic?Ch. 10 - Prob. 57PCh. 10 - Prob. 58PCh. 10 - When heated with H2SO4, both...Ch. 10 - What is the major product obtained from the...Ch. 10 - Write the appropriate reagent over each arrow.Ch. 10 - What alkenes would you expect to be obtained from...Ch. 10 - Prob. 63PCh. 10 - Prob. 64PCh. 10 - When deuterated phenanthrene oxide undergoes a...Ch. 10 - An unknown alcohol with a molecular formula of...Ch. 10 - Explain why the acid-catalyzed dehydration of an...Ch. 10 - Prob. 68PCh. 10 - Prob. 69PCh. 10 - Propose a mechanism for the following reaction:Ch. 10 - What product would be formed if the four-membered...Ch. 10 - Which of the following ethers would be obtained in...Ch. 10 - Using the given starting material any necessary...Ch. 10 - Prob. 74PCh. 10 - When 3-methyl-2-butanol is heated with...Ch. 10 - Draw structures for compounds AF.Ch. 10 - Propose a mechanism for each of the following...Ch. 10 - How could you synthesize isopropyl propyl ether,...Ch. 10 - When ethyl ether is heated with excess HI for...Ch. 10 - When the following seven-membered ring alcohol is...Ch. 10 - Ethylene oxide reacts readily with HO because of...Ch. 10 - Describe how each of the following compounds could...Ch. 10 - Propose a mechanism for each of the following...Ch. 10 - Triethylene glycol is one of the products obtained...Ch. 10 - Prob. 85PCh. 10 - Propose a mechanism for the following reaction:Ch. 10 - Prob. 87PCh. 10 - An ion with a positively charged nitrogen atom in...Ch. 10 - The following reaction takes place several times...Ch. 10 - Prob. 90PCh. 10 - Propose a mechanism for each of the following...Ch. 10 - A vicinal diol has OH groups on adjacent carbons....Ch. 10 - Prob. 93PCh. 10 - Prob. 94PCh. 10 - Two stereoisomers are obtained from the reaction...Ch. 10 - Propose a mechanism for each or the following...Ch. 10 - Triethylenemelamine (TEM) is an antitumor agent....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forwardDraw the major product in each of the following reaction:arrow_forward
- Draw the mechanism for the following Friedel-Craft reaction. AlBr3 Brarrow_forward(a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Consider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT