
(a)
Interpretation:
The primary amine starting material should be identified to form a 4-Methyl-2-pentene via Hofmann degradation.
Concept introduction:
Generally
In the reaction, quaternary ammonium halide can be converted in to quaternary ammonium hydroxide by using aqueous silver oxide.
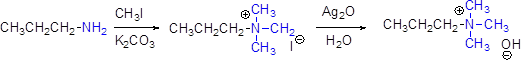
Hofmann elimination:
Quaternary ammonium ion undergoes elimination when using strong base like hydroxide ion this reaction is called as Hofmann elimination.
In the reaction, proton abstraction is takes place in β- carbon atom, which is having more number of hydrogen.

(b)
Interpretation:
The primary amine starting material should be identified to form a 3-Methyl-l-butene via Hofmann degradation.
Concept introduction:
Hofmann elimination:
Quaternary ammonium ion undergoes elimination when using strong base like hydroxide ion this reaction is called as Hofmann elimination.
In the reaction, proton abstraction is takes place in β- carbon atom, which is having more number of hydrogen.

(c)
Interpretation:
The secondary amine starting material should be identified to form a 2-Methyl-1·3-butadiene via Hofmann degradation.
Concept introduction:
Hofmann elimination:
Quaternary ammonium ion undergoes elimination when using strong base like hydroxide ion this reaction is called as Hofmann elimination.
In the reaction, proton abstraction is takes place in β- carbon atom, which is having more number of hydrogen.

Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
- Provide the reasonable steps to achieve the following synthesis.arrow_forwardWhen anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forward
- For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forwardConsider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forward
- For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forwardGiven the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


