
EBK CHEMISTRY:CENTRAL SCIENCE
14th Edition
ISBN: 9780134554570
Author: Brown
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 76E
Indicate which of the following statement regarding the kinetic-molecular theory of gases are correct.
- The average kinetic energy of a collection of gas molecules at a given temperature is proportional to
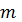
 1/2.
1/2. - The gas molecules are assumed to exert no forces on each other.
- All the molecules of a gas at a given temperature have the same kinetic energy.
- The volume of the gas molecules is negligible in comparison to the total volume in which the gas is contained.
- All gas molecules move with the same speed if they are at the same temperature.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Problem 54, could you please explain it in detail? Thank you! Step by step, I'm really confused, so please don't make it overly complex. My question is to visually draw it out and demonstrate it to me; I'm confused about that problem, please (not just in words) but demonstrate it to me in all due essence (visually) with descriptions.
Explain the types of electromeric effects +E and -E.
Briefly describe the electromeric effect (Organic Chemistry)
Chapter 10 Solutions
EBK CHEMISTRY:CENTRAL SCIENCE
Ch. 10.2 - Prob. 10.1.1PECh. 10.2 -
Gallium melts just above room temperature...Ch. 10.2 - Prob. 10.2.1PECh. 10.2 - Prob. 10.2.2PECh. 10.3 - Prob. 10.3.1PECh. 10.3 - Prob. 10.3.2PECh. 10.4 - Prob. 10.4.1PECh. 10.4 - Prob. 10.4.2PECh. 10.4 - Prob. 10.5.1PECh. 10.4 - Prob. 10.5.2PE
Ch. 10.4 - Prob. 10.6.1PECh. 10.4 - Prob. 10.6.2PECh. 10.5 - Prob. 10.7.1PECh. 10.5 - Prob. 10.7.2PECh. 10.5 - Prob. 10.8.1PECh. 10.5 - Prob. 10.8.2PECh. 10.5 - Prob. 10.9.1PECh. 10.5 - Prob. 10.9.2PECh. 10.6 - Prob. 10.10.1PECh. 10.6 - Prob. 10.10.2PECh. 10.6 - Prob. 10.11.1PECh. 10.6 - Prob. 10.11.2PECh. 10.7 - Prob. 10.12.1PECh. 10.7 - Prob. 10.12.2PECh. 10.8 - Fill in the blanks for the following statement:...Ch. 10.8 - Prob. 10.13.2PECh. 10.8 - Prob. 10.14.1PECh. 10.8 - Prob. 10.14.2PECh. 10.9 - Calculate the pressure of a 2975-mol sample of N2...Ch. 10.9 - Prob. 10.15.2PECh. 10 - Prob. 1DECh. 10 - Prob. 1ECh. 10 - Prob. 2ECh. 10 - Consider the sample of gas depicted here_ What...Ch. 10 - Imagine that the reaction 2CO(g)+O2(g)2CO(g)...Ch. 10 - Suppose you have a fixed amount of an ideal gas at...Ch. 10 - Prob. 6ECh. 10 - Prob. 7ECh. 10 - Prob. 8ECh. 10 - Prob. 9ECh. 10 - Prob. 10ECh. 10 -
10.11 A thin glass tube 1 m long is filled with...Ch. 10 -
10.12 The graph below shows the change in...Ch. 10 - Prob. 13ECh. 10 - Prob. 14ECh. 10 - Prob. 15ECh. 10 - Prob. 16ECh. 10 - Prob. 17ECh. 10 - a. The compound 1-iodododecane is a nonvolatile...Ch. 10 - Prob. 19ECh. 10 - Prob. 20ECh. 10 - Prob. 21ECh. 10 - Prob. 22ECh. 10 - Prob. 23ECh. 10 - Prob. 24ECh. 10 - You have a gas at 25C confined to a cylinder with...Ch. 10 - Prob. 26ECh. 10 - Prob. 27ECh. 10 - Nitrogen and hydrogen gases react to form ammonia...Ch. 10 -
10.29
a. What conditions are represented by the...Ch. 10 - Prob. 30ECh. 10 - Prob. 31ECh. 10 - Prob. 32ECh. 10 - Prob. 33ECh. 10 - Prob. 34ECh. 10 - Prob. 35ECh. 10 - Prob. 36ECh. 10 - Calculate the number of molecules in deep breath...Ch. 10 - If the pressure exerted by ozone, O3, in the...Ch. 10 - A scuba diver’s tank contain 0.29 kg of O2...Ch. 10 - Prob. 40ECh. 10 - Prob. 41ECh. 10 - Prob. 42ECh. 10 - Chlorine is widely used to purify municipal water...Ch. 10 - Many gases are shipped in high-pressure...Ch. 10 - Prob. 45ECh. 10 - Prob. 46ECh. 10 - Rank the following gases from least to denser at...Ch. 10 - Prob. 48ECh. 10 - Prob. 49ECh. 10 - Prob. 50ECh. 10 - Prob. 51ECh. 10 - Prob. 52ECh. 10 - Prob. 53ECh. 10 - Prob. 54ECh. 10 - Magnesium can be used as a ‘getter” in evacuated...Ch. 10 - Prob. 56ECh. 10 - The metabolic oxidation of glucose, C6H12O6, in...Ch. 10 - Prob. 58ECh. 10 - Prob. 59ECh. 10 - Prob. 60ECh. 10 - Consider the apparatus shown in the following...Ch. 10 - Prob. 62ECh. 10 - A mixture containing 0.75 mol He(g), 0.330 mol...Ch. 10 - A deep-sea diver uses a gas cylinder with a volume...Ch. 10 - Prob. 65ECh. 10 - Prob. 66ECh. 10 - Prob. 67ECh. 10 - Prob. 68ECh. 10 - Prob. 69ECh. 10 - Prob. 70ECh. 10 - Prob. 71ECh. 10 - Prob. 72ECh. 10 - Prob. 73ECh. 10 - Prob. 74ECh. 10 - Prob. 75ECh. 10 - Indicate which of the following statement...Ch. 10 - Prob. 77ECh. 10 - Prob. 78ECh. 10 - Prob. 79ECh. 10 - Suppose you have two 1-L flasks, one containing N2...Ch. 10 - Prob. 81ECh. 10 -
10.8
Place the following gases in order of...Ch. 10 - Prob. 83ECh. 10 - Prob. 84ECh. 10 - Prob. 85ECh. 10 - Prob. 86ECh. 10 - Prob. 87ECh. 10 - Prob. 88ECh. 10 - Prob. 89ECh. 10 - Prob. 90ECh. 10 - Prob. 91ECh. 10 - Prob. 92ECh. 10 - Prob. 93ECh. 10 - Prob. 94ECh. 10 - Prob. 95ECh. 10 - Prob. 96ECh. 10 - Prob. 97AECh. 10 - A gas bubble with a volume of 1.0 mm3 originates...Ch. 10 - A 15.0-L tank is filled with helium gas at a...Ch. 10 - Prob. 100AECh. 10 - Prob. 101AECh. 10 - Prob. 102AECh. 10 - Prob. 103AECh. 10 - Prob. 104AECh. 10 - Prob. 105AECh. 10 - Prob. 106AECh. 10 - Prob. 107AECh. 10 - Prob. 108AECh. 10 - Prob. 109AECh. 10 - The density of gas of unknown molar mass was...Ch. 10 - A glass vessel fitted with a stopcock valve has a...Ch. 10 - Prob. 112AECh. 10 -
10.113 consider the following gases. All at STP:...Ch. 10 - Prob. 114AECh. 10 - Prob. 115AECh. 10 - Prob. 116AECh. 10 - Prob. 117AECh. 10 - Prob. 118IECh. 10 - Prob. 119IECh. 10 - Prob. 120IECh. 10 -
10.121 A 4.00-g sample of a mixture of CaO and...Ch. 10 - Prob. 122IECh. 10 - Prob. 123IECh. 10 - Chlorine dioxide gas (CIO2) is used as a...Ch. 10 - Natural gas is very abundant in many Middle...Ch. 10 - Prob. 126IECh. 10 - Prob. 127IE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. (CH3)2NH, TSOH Drawingarrow_forwardSo, the first image is what I'm trying to understand regarding my approach. The second image illustrates my teacher's method, and the third image includes my notes on the concepts behind these types of problems.arrow_forwardHAND DRAWarrow_forward
- Draw a mental model for calcium chloride mixed with sodium phosphatearrow_forwardhere is my question (problem number 20) please explain to me thanks!arrow_forwardThe bromination of anisole is an extremely fast reaction. Complete the resonance structures of the intermediate arenium cation for the reaction (Part 1), and then answer the question that follows (Part 2).arrow_forward
- Drawing of 3-fluro-2methylphenolarrow_forwardWhich compound(s) will be fully deprotonated (>99%) by reaction with one molar equivalent of sodium hydroxide? I, II, III I, || I, III I only II, III SH | H3C-C=C-H || III NH2arrow_forwardWill NBS (and heat or light) work for this reaction, or do we have to use Br2?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHERChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHERChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Step by Step Stoichiometry Practice Problems | How to Pass ChemistryMole Conversions Made Easy: How to Convert Between Grams and Moles; Author: Ketzbook;https://www.youtube.com/watch?v=b2raanVWU6c;License: Standard YouTube License, CC-BY