
MOLECULAR NATURE OF MATTER 7/E LL W/AC
7th Edition
ISBN: 9781119664796
Author: JESPERSEN
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 10, Problem 61RQ
Interpretation Introduction
Interpretation:
The volume of carbon dioxide in milliliters at a given temperature and pressure that could be formed in the combustion of carbon monoxide is to be calculated.
Concept Introduction:
The effect of temperature and pressure on the volume of a gas is given by an equation called the ideal gas equation. It is expressed as follows:
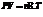
Here, 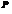 is the pressure,
is the pressure, 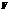 is the volume,
is the volume,  is the number of moles,
is the number of moles, 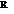 is the gas constant, and
is the gas constant, and 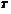 is the temperature.
is the temperature.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
b) Certain cyclic compounds are known to be conformationally similar to carbohydrates, although they are not
themselves carbohydrates. One example is Compound C shown below, which could be imagined as adopting
four possible conformations. In reality, however, only one of these is particularly stable. Circle the conformation
you expect to be the most stable, and provide an explanation to justify your choice. For your explanation to be
both convincing and correct, it must contain not only words, but also "cartoon" orbital drawings contrasting the
four structures.
Compound C
Possible conformations (circle one):
Дет
Lab Data
The distance entered is out of the expected range.
Check your calculations and conversion factors.
Verify your distance. Will the gas cloud be closer to the cotton ball with HCI or NH3?
Did you report your data to the correct number of significant figures?
- X
Experimental Set-up
HCI-NH3
NH3-HCI
Longer Tube
Time elapsed (min)
5 (exact)
5 (exact)
Distance between cotton balls (cm)
24.30
24.40
Distance to cloud (cm)
9.70
14.16
Distance traveled by HCI (cm)
9.70
9.80
Distance traveled by NH3 (cm)
14.60
14.50
Diffusion rate of HCI (cm/hr)
116
118
Diffusion rate of NH3 (cm/hr)
175.2
175.2
How to measure distance and calculate rate
For the titration of a divalent metal ion (M2+) with EDTA, the stoichiometry of the reaction is typically:
1:1 (one mole of EDTA per mole of metal ion)
2:1 (two moles of EDTA per mole of metal ion)
1:2 (one mole of EDTA per two moles of metal ion)
None of the above
Chapter 10 Solutions
MOLECULAR NATURE OF MATTER 7/E LL W/AC
Ch. 10 - Prob. 1PECh. 10 - Prob. 2PECh. 10 - Prob. 3PECh. 10 - Prob. 4PECh. 10 - Prob. 5PECh. 10 - Prob. 6PECh. 10 - Prob. 7PECh. 10 - Prob. 8PECh. 10 - Prob. 9PECh. 10 - Prob. 10PE
Ch. 10 - Practice Exercise 10.11 How many grams of argon...Ch. 10 - Prob. 12PECh. 10 - Practice Exercise 10.13
The label on a cylinder of...Ch. 10 - A glass bulb is found to have a volume of 544.23...Ch. 10 - Sulfur dioxide is a gas that has been used in...Ch. 10 - Radon, a radioactive gas, is formed in one step of...Ch. 10 - Practice Exercise 10.17
A gaseous compound of...Ch. 10 - A compound composed of only carbon and hydrogen...Ch. 10 - Carbon disulfide is an extremely flammable liquid....Ch. 10 - In one lab, thegas-collecting apparatus used a gas...Ch. 10 - The explosive PETN, pentaerythritoltetranitrate,...Ch. 10 - Prob. 22PECh. 10 - Suppose you prepared a sample of nitrogen and...Ch. 10 - A 2.50 L sample of methane was collected over...Ch. 10 - Suppose a mixture containing 2.15 g H2 and 34.0 g...Ch. 10 - Sulfur dioxide and oxygen react according to the...Ch. 10 - Bromine has two isotopes with masses of 78.9 and...Ch. 10 - The hydrogen halide gases all have the same...Ch. 10 - Prob. 1RQCh. 10 - Prob. 2RQCh. 10 - Prob. 3RQCh. 10 - Prob. 4RQCh. 10 - Prob. 5RQCh. 10 - Prob. 6RQCh. 10 - Prob. 7RQCh. 10 - What is meant by an ideal gas? Under what...Ch. 10 - Prob. 9RQCh. 10 - Prob. 10RQCh. 10 - Prob. 11RQCh. 10 - Prob. 12RQCh. 10 - Prob. 13RQCh. 10 - Prob. 14RQCh. 10 - Prob. 15RQCh. 10 - Prob. 16RQCh. 10 - Prob. 17RQCh. 10 - Prob. 18RQCh. 10 - Prob. 19RQCh. 10 - Prob. 20RQCh. 10 - Prob. 21RQCh. 10 - Prob. 22RQCh. 10 - Prob. 23RQCh. 10 - Prob. 24RQCh. 10 - Prob. 25RQCh. 10 - Prob. 26RQCh. 10 - Prob. 27RQCh. 10 - Prob. 28RQCh. 10 - Prob. 29RQCh. 10 - Prob. 30RQCh. 10 - What does a small value for the van der Waals...Ch. 10 - Which of the molecules below has the larger value...Ch. 10 - Under the same conditions of T and V, why is the...Ch. 10 - Prob. 34RQCh. 10 - Carry out the following unit conversions: (a) 1.26...Ch. 10 - Prob. 36RQCh. 10 - Prob. 37RQCh. 10 - 10.38 What is the pressure in atm of each of the...Ch. 10 - 10.39 An open-end manometer containing mercury was...Ch. 10 - Prob. 40RQCh. 10 - Prob. 41RQCh. 10 - An open-end mercury manometer was connected to a...Ch. 10 - Prob. 43RQCh. 10 - 10.44 Suppose a gas is in a vessel connected to...Ch. 10 - Prob. 45RQCh. 10 - Prob. 46RQCh. 10 - Prob. 47RQCh. 10 - Prob. 48RQCh. 10 - Prob. 49RQCh. 10 - Prob. 50RQCh. 10 - A sample of helium at a pressure of 74$ torr and...Ch. 10 - When a sample of neon with a volume of 648 mL and...Ch. 10 - What must be the new volume of a sample of...Ch. 10 - When 286 mL of oxygen at 741 torr and 18.0C was...Ch. 10 - A sample of argon with a volume of 6.18 L, a...Ch. 10 - Prob. 56RQCh. 10 - How many milliliters of O2 are consumed in the...Ch. 10 - How many milliliters of oxygen are required to...Ch. 10 - *10.59 How many milliliters of measured at and...Ch. 10 - How many milliliters of H2O vapor, measured at...Ch. 10 - Prob. 61RQCh. 10 - Prob. 62RQCh. 10 - Prob. 63RQCh. 10 - Prob. 64RQCh. 10 - Prob. 65RQCh. 10 - Prob. 66RQCh. 10 - Prob. 67RQCh. 10 - Prob. 68RQCh. 10 - Prob. 69RQCh. 10 - 10.70 Methane is formed in landfills by the action...Ch. 10 - A chemist isolated a gas in a glass bulb with a...Ch. 10 - Prob. 72RQCh. 10 - 10.73 To three significant figures, calculate the...Ch. 10 - To three significant figures, calculate the...Ch. 10 - 10.75 What density does oxygen have at and 742...Ch. 10 - At 748.0 torr and 20.65C, what is the density of...Ch. 10 - The explosive PETN, pentaerythritol tetranitrate,...Ch. 10 - TNT, trinitrotoluene, is an explosive that can...Ch. 10 - Propylene, C3H6, reacts with hydrogen under...Ch. 10 - Nitric acid is formed when NO2 is dissolved in...Ch. 10 - A mixture of gases contains 315 torr N2, 275 torr...Ch. 10 - Prob. 82RQCh. 10 - A 1.00 L container was filled by pumping into it...Ch. 10 - A special gas mixture, BAR 97 High without NO, is...Ch. 10 - Prob. 85RQCh. 10 - Prob. 86RQCh. 10 - A 22.4 L container at 0C contains 0.300 mol N2,...Ch. 10 - A mixture of N2,O2,andCO2 Has a total pressure of...Ch. 10 - A 0.200 mol sample of a mixture of N2 and CO2 with...Ch. 10 - A sample of carbon monoxide was prepared and...Ch. 10 - Prob. 91RQCh. 10 - What volume of wet oxygen would you have to...Ch. 10 - Prob. 93RQCh. 10 - Prob. 94RQCh. 10 - Prob. 95RQCh. 10 - 10.96 For the gases which gas will effuse the...Ch. 10 - Prob. 97RQCh. 10 - Prob. 98RQCh. 10 - Uranium hexafluoride is a white solid that readily...Ch. 10 - Prob. 100RQCh. 10 - Prob. 101RQCh. 10 - A typical automobile has a weight of approximately...Ch. 10 - *10.103 Suppose you were planning to move a house...Ch. 10 - Prob. 104RQCh. 10 - Two flasks (which we will refer to as flask 1 and...Ch. 10 - *10.106 A bubble of air escaping from a divers...Ch. 10 - *10.107 In a diesel engine, the fuel is ignited...Ch. 10 - *10.108 Early one cool (60.0F) morning you start...Ch. 10 - Prob. 109RQCh. 10 - *10.110 A mixture was prepared in a 0.500 L...Ch. 10 - *10.111 A student collected 18.45 mL of H2 over...Ch. 10 - *10.112 A mixture of gases is prepared from 87.5 g...Ch. 10 - 10.113 A gas was found to have a density of...Ch. 10 - *10.114 In one analytical procedure for...Ch. 10 - Prob. 115RQCh. 10 - Prob. 116RQCh. 10 - Prob. 117RQCh. 10 - The odor of a rotten egg is caused by hydrogen...Ch. 10 - Chlorine reacts with sulfite ion to give sulfate...Ch. 10 - *10.120 In an experiment designed to prepare a...Ch. 10 - Carbon dioxide can be made in the lab by the...Ch. 10 - 10.122 Boron forms a variety of unusual compounds...Ch. 10 - Prob. 123RQCh. 10 - Carbon dioxide is implicated in global warming....Ch. 10 - Prob. 125RQCh. 10 - One of the that is implicated in decreasing the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning