
Concept explainers
In the mass spectrum of the following compounds, which would be more intense: the peak at m/z = 57 or the peak at m/z = 71?
- a. 3-methylpentane
- b. 2-methylpentane
Interpretation
The tallest peak should be identified.
Explanation of Solution
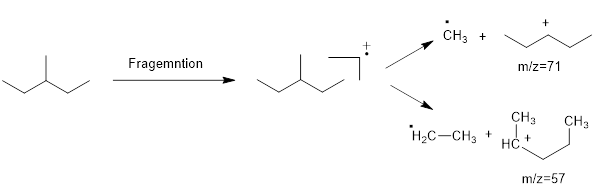
Mass fragmentation of 3-methyl pentane: In this molecule will be more adopted to lose an ethyl radical forming a secondary carbocation and a primary radical than a methyl radical forming secondary carbocation and methyl radical. In addition, 3-methylpentane ha undergoes for two path ways to lose an ethyl radical,
Therefore, the peak at
Mass fragmentation of 2-methyl pentane: The 2-methylpentane has undergoes for pathways to lose a methyl radical in each pathway and it cannot form a secondary carbocation by losing an ethyl radical. Loss of an ethyl radical would form a primary carbocation and a primary radical. Therefore, it will be more adopt to lose a methyl radical than an ethyl radical, the corresponding peak at
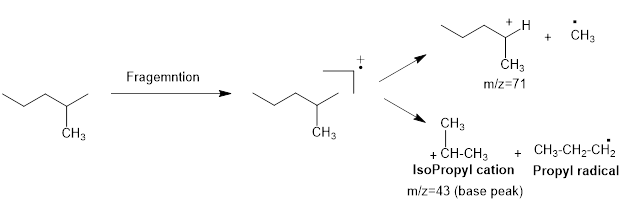
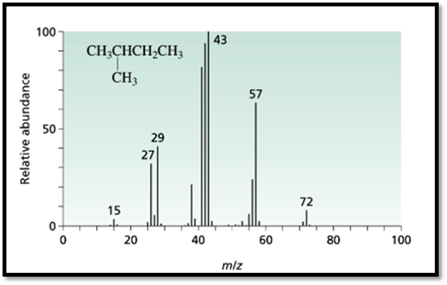
Figure 1
The 3-methyl pentane molecular peak at the m/e=71 has more intense then the peak at
Want to see more full solutions like this?
Chapter 10 Solutions
Essential Organic Chemistry (3rd Edition)
- Name the following molecules with IUpacarrow_forwardWhat is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forward
- How to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

