
Concept explainers
In the mass spectrum of the following compounds, which would be more intense: the peak at m/z = 57 or the peak at m/z = 71?
- a. 3-methylpentane
- b. 2-methylpentane
Interpretation
The tallest peak should be identified.
Explanation of Solution
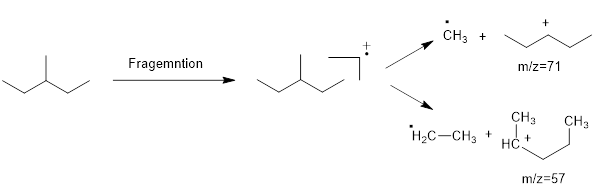
Mass fragmentation of 3-methyl pentane: In this molecule will be more adopted to lose an ethyl radical forming a secondary carbocation and a primary radical than a methyl radical forming secondary carbocation and methyl radical. In addition, 3-methylpentane ha undergoes for two path ways to lose an ethyl radical,
Therefore, the peak at
Mass fragmentation of 2-methyl pentane: The 2-methylpentane has undergoes for pathways to lose a methyl radical in each pathway and it cannot form a secondary carbocation by losing an ethyl radical. Loss of an ethyl radical would form a primary carbocation and a primary radical. Therefore, it will be more adopt to lose a methyl radical than an ethyl radical, the corresponding peak at
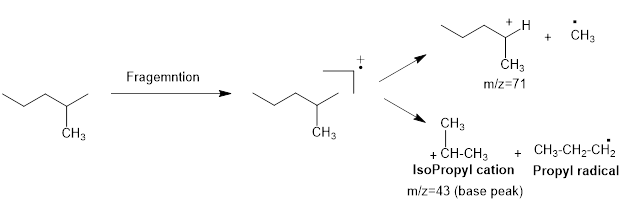
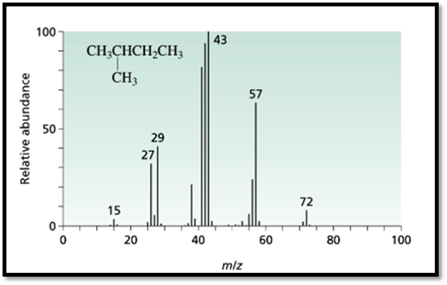
Figure 1
The 3-methyl pentane molecular peak at the m/e=71 has more intense then the peak at
Want to see more full solutions like this?
Chapter 10 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
- Indicate the coordination forms of Si in silicates.arrow_forwardBriefly indicate the structure and bonding of silicates.arrow_forward4 Part C Give the IUPAC name and a common name for the following ether: Spell out the full names of the compound in the indicated order separated by a comma.arrow_forward
- Try: Draw possible resonance contributing structures for the following organic species: CH3CH2NO2 [CH2CHCH2] [CH2CHCHO] [CH2CHCH2] [CH2CHNH2]arrow_forwardComplete the following synthesis. (d). H+ ง сarrow_forwardCan the target compound be efficiently synthesized in good yield from the substituted benzene of the starting material? If yes, draw the synthesis. Include all steps and all reactants.arrow_forward
- This is a synthesis question. Why is this method wrong or worse than the "correct" method? You could do it thiss way, couldn't you?arrow_forwardTry: Draw the best Lewis structure showing all non-bonding electrons and all formal charges if any: (CH3)3CCNO NCO- HN3 [CH3OH2]*arrow_forwardWhat are the major products of the following reaction? Draw all the major products. If there are no major products, then there is no reaction that will take place. Use wedge and dash bonds when necessary.arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

