
Concept explainers
In the mass spectrum of the following compounds, which would be more intense: the peak at m/z = 57 or the peak at m/z = 71?
- a. 3-methylpentane
- b. 2-methylpentane
Interpretation
The tallest peak should be identified.
Explanation of Solution
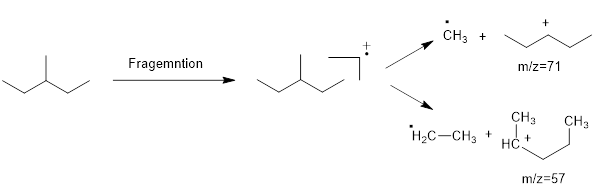
Mass fragmentation of 3-methyl pentane: In this molecule will be more adopted to lose an ethyl radical forming a secondary carbocation and a primary radical than a methyl radical forming secondary carbocation and methyl radical. In addition, 3-methylpentane ha undergoes for two path ways to lose an ethyl radical,
Therefore, the peak at
Mass fragmentation of 2-methyl pentane: The 2-methylpentane has undergoes for pathways to lose a methyl radical in each pathway and it cannot form a secondary carbocation by losing an ethyl radical. Loss of an ethyl radical would form a primary carbocation and a primary radical. Therefore, it will be more adopt to lose a methyl radical than an ethyl radical, the corresponding peak at
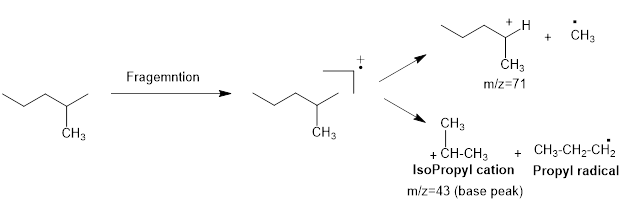
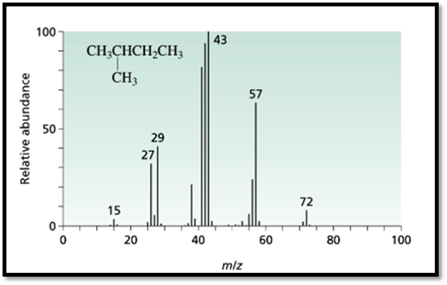
Figure 1
The 3-methyl pentane molecular peak at the m/e=71 has more intense then the peak at
Want to see more full solutions like this?
Chapter 10 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
- 10 Question (1 point) Draw curved arrow notation to indicate the proton transfer between NaOH and CH3CO₂H. 2nd attempt :0- H See Periodic Table See Hint Draw the products of the proton transfer reaction. Don't add a + sign between the products.arrow_forwardProvide steps and explanation please.arrow_forwardProvide steps to name and label for understanding.arrow_forward
- Provide the IUPAC name of the following molecule. Don't forget to include the proper stereochemistry where appropriate.arrow_forward3. 2. 1. On the graph below, plot the volume of rain in milliliters versus its height in centimeters for the 400 mL beaker. Draw a straight line through the points and label it "400 mL beaker." Volume (mL) 400 350 300 250 200 150 750 mL Florence Volume Versus Height of Water 400 mL beaker 100 50 0 0 2 3 4 5 Height (cm) 6 7 8 9 10 Explain why the data points for the beaker lie roughly on a straight line. What kind of relationship is this? How do you know? (see page 276 text) the design of the beaker is a uniform cylinder the volume of liquid increases evenly with its height resulting in a linear relationship. What volume would you predict for 10.0 cm of water? Explain how you arrived at your answer. Use the data table and the graph to assist you in answering the question. 4. Plot the volume of rain in milliliters versus its height in centimeters for the 250 mL Florence flask on the same graph. Draw a best-fit curve through the points and label it "250 mL Florence flask." oke camearrow_forwardShow work. Don't give Ai generated solutionarrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

