
Concept explainers
(a)
Interpretation:
The electronic configuration of Manganese has to be given.
Concept Introduction:
Aufbau’s principle:
Aufbau’s principle states that electrons are filled into atomic orbitals in the increasing order of orbital energy level. According to the Aufbau principle the available atomic orbitals with the lowest energy levels are occupied before those with higher energy levels.
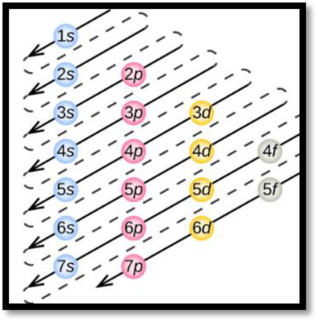
Figure 1
(b)
Interpretation:
The electronic configuration of Krypton has to be given.
Concept Introduction:
Refer to part (a).
(c)
Interpretation:
The electronic configuration of Gallium has to be given.
Concept Introduction:
Refer to part (a).
(d)
Interpretation:
The electronic configuration of Boron has to be given.
Concept Introduction:
Refer to part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Foundations of College Chemistry 15e Binder Ready Version + WileyPLUS Registration Card
- 19.57 Using one of the reactions in this chapter, give the correct starting material (A-L) needed to produce each structure (a-f). Name the type of reaction used. (b) ہ مرد (d) HO (c) དང་ ་་ཡིན་ད་དང་ (f) HO Br B D of oli H J Br K C 人 ↑arrow_forwardInductive effect (+I and -I) in benzene derivatives.arrow_forward7. Helparrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





