
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 37P
Label the
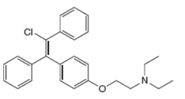
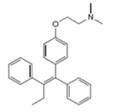
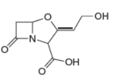
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Write the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophen
For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. 
How to draw the reaction mechasnism below
Chapter 10 Solutions
Organic Chemistry (6th Edition)
Ch. 10.1 - Prob. 1PCh. 10.2 - Problem 10.2 How many degrees of unsaturation are...Ch. 10.3 - Give the IUPAC name for each alkene. abcdeCh. 10.3 - Give the IUPAC name for each polyfunctional...Ch. 10.3 - Prob. 9PCh. 10.6 - Linolenic acidTable 10.2 and stearidonic acid are...Ch. 10.7 - Prob. 12PCh. 10.9 - Problem 10.13 What product is formed when each...Ch. 10.9 - Prob. 14PCh. 10.10 - Problem 10.15 Draw the products formed when each...
Ch. 10.10 - Prob. 16PCh. 10.10 - Prob. 17PCh. 10.10 - Addition of HBr to which of the following alkenes...Ch. 10.11 - Problem 10.19 Draw the products, including...Ch. 10.11 - Prob. 20PCh. 10.12 - Problem 10.21 What two alkenes give rise to each...Ch. 10.12 - Prob. 22PCh. 10.13 - Problem 10.23 Draw the products of each reaction,...Ch. 10.14 - Problem 10.24 Draw all stereoisomers formed in...Ch. 10.15 - Prob. 25PCh. 10.16 - Problem 10.26 What alkylborane is formed from...Ch. 10.16 - Draw the products formed when each alkene is...Ch. 10.16 - What alkene can be used to prepare each alcohol as...Ch. 10.16 - Prob. 29PCh. 10.17 - Draw the products of each reaction using the two...Ch. 10.18 - Problem 10.31 Devise a synthesis of each compound...Ch. 10 - Give the IUPAC name for each compound. a.b.Ch. 10 - a Label the carbon-carbon double bond in A as E or...Ch. 10 - Prob. 34PCh. 10 - 10.35 Calculate the number of degrees of...Ch. 10 - Prob. 36PCh. 10 - Label the alkene in each drug as E or Z....Ch. 10 - Give the IUPAC name for each compound. a. c. e. b....Ch. 10 - Prob. 39PCh. 10 - 10.40 (a) Draw all possible stereoisomers of, and...Ch. 10 - Prob. 41PCh. 10 - 10.42 Now that you have learned how to name...Ch. 10 - Prob. 43PCh. 10 - Prob. 44PCh. 10 - Prob. 45PCh. 10 - Draw the products formed when (CH3)2C=CH2 is...Ch. 10 - What alkene can be used to prepare each alkyl...Ch. 10 - Prob. 48PCh. 10 - Draw the constitutional isomer formed in each...Ch. 10 - Prob. 50PCh. 10 - Draw all stereoisomers formed in each reaction. a....Ch. 10 - Draw the products of each reaction, including...Ch. 10 - Prob. 53PCh. 10 - Draw a stepwise mechanism that shows how all three...Ch. 10 - Less stable alkenes can be isomerized to more...Ch. 10 - Prob. 60PCh. 10 - Prob. 61PCh. 10 - Bromoetherification, the addition of the elements...Ch. 10 - Devise a synthesis of each product from the given...Ch. 10 - 10.65 Draw a synthesis of each compound from...
Additional Science Textbook Solutions
Find more solutions based on key concepts
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the following molecules with IUpacarrow_forwardWhat is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forward
- How to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY