
Interpretation:
The resonance structure of the radical should be identified for the given molecule.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical
Answer to Problem 23PP
Answer
An unpaired electron occupies the allylic position and in is involving in resonance. The arrow pattern of radical resonance is given below (a).

An unpaired electron occupies the allylic position and in is involving in resonance. The arrow pattern of radical resonance is given below (b).
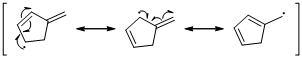
An unpaired electron occupies the allylic position and in is involving in resonance. The arrow pattern of radical resonance is given below (c).
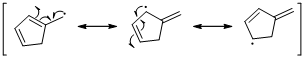
Unpaired electrons resonance with allylic double bonds. The arrow pattern of radical resonance is given below (d).
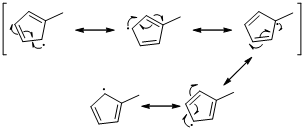
Unpaired electrons resonance with allylic double bonds. The arrow pattern of radical resonance is given below (f)
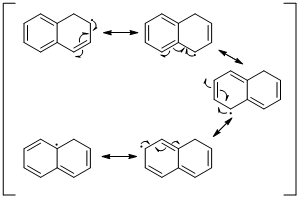
Explanation of Solution
The radical resonance structure of the given molecule
The radical resonance structure of the molecule is given below.

An unpaired electron occupies the allylic position and in is involving in resonance. Here three fishhook arrows were used to show the resonance.
The radical resonance structure of the molecule is given below.

An unpaired electron occupies the allylic position and in is involving in resonance. Here three fishhook arrows were used to show the resonance. The resulting resonance structure exhibits an unpaired electrons, the unpaired electrons allylic to another π bond that also involving resonance. Again three fishhook arrows were used for the resonance.
The radical resonance structure of the molecule is given below.
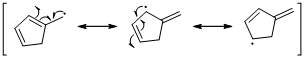
An unpaired electron occupies the allylic position and in is involving in resonance. Here three fishhook arrows were used to show the resonance. The resulting resonance structure exhibits an unpaired electrons, the unpaired electrons allylic to another π bond that also involving resonance. Again three fishhook arrows were used for the resonance.
The radical resonance structure of the molecule is given below.
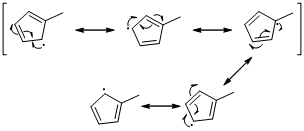
An unpaired electron occupies the allylic position and in is involving in resonance. Here three fishhook arrows were used to show the resonance. The resulting resonance structure exhibits an unpaired electrons, the unpaired electrons allylic to another π bond that also involving resonance. Again three fishhook arrows were used for the resonance. Similarly the five resonances structure is exist in same manner as shown above structure.
The radical resonance structure of the molecule is given below.
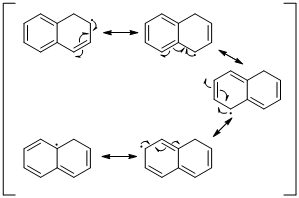
An unpaired electron occupies the allylic position and in is involving in resonance. Here three fishhook arrows were used to show the resonance. The resulting resonance structure exhibits an unpaired electrons, the unpaired electrons allylic to another π bond that also involving resonance. Again three fishhook arrows were used for the resonance. Similarly the five resonances structure is exist in same manner as shown above structure.
Conclusion
The resonance structure of the radical for the given molecule is found.
Want to see more full solutions like this?
Chapter 10 Solutions
ORGANIC CHEMISTRY 1 TERM ACCESS
- 8. Choose the compound that will produce the spectrum below and assign the signals to the corresponding protons. 2 4 3 ō (ppm) OH 4 6 6 СОН 2 1 0arrow_forward7. Assign all of the protons on the spectrum below. A B 2 C E 2 1 3 6 4 3 2 1 0arrow_forwarde. If (3R,4R)-3,4-dichloro-2,5-dimethylhexane and (3R,4S)-3,4-dichloro-2,5-dimethylhexane are in a solution at the same concentration, would this solution be expected to rotate plane polarized light (that is, be optically active)? Please provide your reasoning for your answer. [If you read this problem carefully, you will not need to draw out the structures to arrive at your answer...]arrow_forward
- 1. How many neighbors does the proton that produces the multiplet below have? 2. 3. اللـ Draw a partial structure from the multiplet below. (The integration of the multiplet is 6) M Using the additivity constants found in appendix G of your lab manual, calculate the approximate chemical shifts of the protons indicated below. (Show your work!!!) B A Br SHarrow_forward1) Suppose 0.1 kg ice at 0°C (273K) is in 0.5kg water at 20°C (293K). What is the change in entropy of the ice as it melts at 0°? To produce the original "water gas" mixture, carbon (in a combustible form known as coke) is reacted with steam: 131.4 kJ + H20(g) + C(s) → CO(g) + H2(g) From this information and the equations in the previous problem, calculate the enthalpy for the combustion or carbon to form carbon dioxide. kindly show me how to solve this long problem. Thanksarrow_forward4. An 'H-NMR of a compound is acquired. The integration for signal A is 5692 and the integration for signal B is 25614. What is the simplest whole number ratio of protons for signals A and B? (Show your work!!!) 5. Assign the carbons in the NMR below as either carbonyl, aromatic, or alkyl. 200 150 100 50 ō (ppm) 1arrow_forward
- Speaking of composite materials, indicate the correct option:(A). Composite materials can only be: metal-polymer or polymer-polymer.(B). Composite materials can be made up of particles, but not fibers or sheets.(C). When the reinforcing particles are uniformly distributed in a composite material, there may be a greater tendency for it to have isotropic properties.(D). None of the above is correct.arrow_forwardIf we are talking about viscoelastic modulus or viscoelastic relaxation modulus in polymers, indicate the correct option.(A). It reports the variation of elastic behavior as a function of time.(B). It is only useful for defining its glass transition temperature.(C). It only allows us to define the polymer degradation temperature.(D). Neither option is correct.arrow_forwardWhen natural light falls perpendicularly on a material A, it has a reflectivity of 0.813%. Indicate the value of the refractive index.arrow_forward
- In piezoelectricity and piezoelectric ceramics, one of the following options is false:(A). Piezoelectricity allows an electrical signal to be transformed into a mechanical one.(B). PbZrO3 is a well-known piezoelectric ceramic.(C). Piezoelectricity and ferroelectricity in general have no relationship.(D). One of the applications of piezoelectricity is sonar.arrow_forward(30 MARKS) Give the major product(s ) formed including relevant stereochemistry or the complete reaction conditions for the following reactions. More than one step may be required for each reaction arrow, in which case the steps must be numbered 1), 2) etc. (2 marks each box) h) i) h) OH i) HO H3PO4, heat 2 Brarrow_forwardNonearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





