
Concept explainers
Predict the geometries of the following species using the VSEPR method: (a) PCl3, (b) CHCl3, (c) SiH4, (d) TeCl4.
(a)
Interpretation: For the given set of molecules the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry: It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
If the molecules (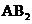 ) have two atoms bonded with the central atom then the molecular geometry by considering the orientation of atoms is linear if it has lone of electron over the central atom then the molecular geometry will be bent.
) have two atoms bonded with the central atom then the molecular geometry by considering the orientation of atoms is linear if it has lone of electron over the central atom then the molecular geometry will be bent.
The molecules of type 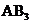 will have shape like trigonal planar and if the molecules has lone pairs then the molecular geometry will be tetrahedral.
will have shape like trigonal planar and if the molecules has lone pairs then the molecular geometry will be tetrahedral.
The molecules of type 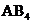 will have shape like tetrahedral, and geometry of type
will have shape like tetrahedral, and geometry of type 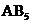 will have trigonal bipyramidal and
will have trigonal bipyramidal and 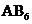 will have shape like octahedral respectively.
will have shape like octahedral respectively.
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
Answer to Problem 10.7QP
(a)
Trigonal pyramidal
Explanation of Solution
To predict: The geometry for the given molecule.
Draw the Lewis structure for the molecule (a)
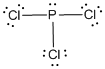
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 26.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 6 has to be subtracted with 26 as each bond contains two electrons with it and there are three bonds in the skeletal structure.
Finally, the 20 electrons got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (a) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type tetrahedral since the is bonded with three chlorine atoms and one lone pair of electron with it.
is bonded with three chlorine atoms and one lone pair of electron with it.
The molecular geometry for the given molecule is trigonal pyramidal due to the presence of one lone pair around the central atom.
(b)
Interpretation: For the given set of molecules the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry: It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
If the molecules (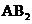 ) have two atoms bonded with the central atom then the molecular geometry by considering the orientation of atoms is linear if it has lone of electron over the central atom then the molecular geometry will be bent.
) have two atoms bonded with the central atom then the molecular geometry by considering the orientation of atoms is linear if it has lone of electron over the central atom then the molecular geometry will be bent.
The molecules of type 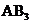 will have shape like trigonal planar and if the molecules has lone pairs then the molecular geometry will be tetrahedral.
will have shape like trigonal planar and if the molecules has lone pairs then the molecular geometry will be tetrahedral.
The molecules of type 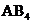 will have shape like tetrahedral, and geometry of type
will have shape like tetrahedral, and geometry of type 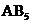 will have trigonal bipyramidal and
will have trigonal bipyramidal and 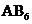 will have shape like octahedral respectively.
will have shape like octahedral respectively.
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
Answer to Problem 10.7QP
(b)
Tetrahedral
Explanation of Solution
To predict: The geometry for the given molecule.
Draw the Lewis structure for the molecule (b)
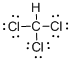
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 26.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 8 has to be subtracted with 26 as each bond contains two electrons with it and there are four bonds in the skeletal structure.
Finally, the 18 electrons got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (b) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type tetrahedral as there is no lone pair of electron over the central metal atom and hence the molecular geometry for the given molecule is also Tetrahedral.
(c)
Interpretation: For the given set of molecules the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry: It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
If the molecules (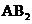 ) have two atoms bonded with the central atom then the molecular geometry by considering the orientation of atoms is linear if it has lone of electron over the central atom then the molecular geometry will be bent.
) have two atoms bonded with the central atom then the molecular geometry by considering the orientation of atoms is linear if it has lone of electron over the central atom then the molecular geometry will be bent.
The molecules of type 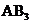 will have shape like trigonal planar and if the molecules has lone pairs then the molecular geometry will be tetrahedral.
will have shape like trigonal planar and if the molecules has lone pairs then the molecular geometry will be tetrahedral.
The molecules of type 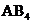 will have shape like tetrahedral, and geometry of type
will have shape like tetrahedral, and geometry of type 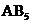 will have trigonal bipyramidal and
will have trigonal bipyramidal and 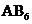 will have shape like octahedral respectively.
will have shape like octahedral respectively.
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
Answer to Problem 10.7QP
(c)
Tetrahedral
Explanation of Solution
To predict: The geometry for the given molecule.
Draw the Lewis structure for the molecule (c)
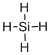
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 8.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 8 has to be subtracted with 8 as each bond contains two electrons with it and there are four bonds in the skeletal structure.
There are no remaining electrons hence all the atoms in the molecules are fulfilled the octet rule that is each atom involves in bonding in order to fill their valence with eight electrons.
Determine the molecular geometry for the molecule (c) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type tetrahedral since central atom does not contain any lone pair of electron with it.
The molecular geometry for the molecule is also tetrahedral as there are four atoms bonded with the central metal atom and there is absence of lone pair of electrons.
(d)
Interpretation: For the given set of molecules the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry: It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
If the molecules (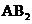 ) have two atoms bonded with the central atom then the molecular geometry by considering the orientation of atoms is linear if it has lone of electron over the central atom then the molecular geometry will be bent.
) have two atoms bonded with the central atom then the molecular geometry by considering the orientation of atoms is linear if it has lone of electron over the central atom then the molecular geometry will be bent.
The molecules of type 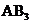 will have shape like trigonal planar and if the molecules has lone pairs then the molecular geometry will be tetrahedral.
will have shape like trigonal planar and if the molecules has lone pairs then the molecular geometry will be tetrahedral.
The molecules of type 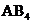 will have shape like tetrahedral, and geometry of type
will have shape like tetrahedral, and geometry of type 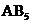 will have trigonal bipyramidal and
will have trigonal bipyramidal and 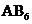 will have shape like octahedral respectively.
will have shape like octahedral respectively.
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
Answer to Problem 10.7QP
(d)
See-saw shaped
Explanation of Solution
To predict: The geometry for the given molecule.
Draw the Lewis structure for the molecule (d)
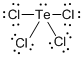
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 34.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 8 has to be subtracted with 8 as each bond contains two electrons with it and there are four bonds in the skeletal structure.
Then the 26 electrons got after the subtractions should be placed over the atoms present in the molecule such that each atom contains eight electrons in the valence shell.
Determine the molecular geometry for the molecule (d) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure shows that it contains five electron domains since it has 4 chlorine atoms and one lone pair with it.
The molecular geometry for the molecule is see-saw shape due to the present of that one lone pair of electron.
Want to see more full solutions like this?
Chapter 10 Solutions
Chemistry
- Need help with 14 and 15. 14. bromobenzene + (CHs),CuLi + THF / -78° followed by water quench is a. toluene else!! b. xylene c. cumene d. styrene e. something 15. When cumene + H,SO, / Na,Cr, 0,/water are mixed (refluxed) what is produced? a. 2-phenylpropanol phenol e. styrene b. benzoic acid c. no reaction!arrow_forwardWhich of the following orbitals intersect or overlap the x-axis in the standard cartesian coordinate system used? (Select ALL correct answers.) Group of answer choices px dxz dx2-y2 py dxy sarrow_forwardWhich of the following sets of elements is not a Dobereiner triad? (Choose the best answer.) Group of answer choices Li-Na-K Al-Ga-In Cr-Mo-W K-Rb-Csarrow_forward
- Don't used Ai solution and don't used hand raitingarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardGive the structure(s) of the product(s) the reaction below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise I'm struggling to see how this reaction will go! I am wondering if it will cycle on itself but I'm not sure how I drew out a decagon but I'm a bit lostarrow_forward
- Give the structure(s) of the product(s) for the reactions below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise .arrow_forwardCalculate the residence time of strontium (Sr2+) in the world ocean, given that the average concentration of strontium in the world’s rivers is approximately 0.87 µmol L-1 (5 pts).arrow_forwardA package contains 1.33lbs of ground round. If it contains 29% fat, how many grams of fat are in the ground? arrow_forward
- How is the resonance structure formed to make the following reaction product. Please hand draw the arrows showing how the electrons move to the correct position. Do not use an AI answer. Please draw it yourself or don't bother.arrow_forwardPart II Calculate λ max of the following compounds using wood ward- Fiecer rules a) b) c) d) e) OH OH dissolved in dioxane Br Br dissolved in methanol. NH₂ OCH 3 OHarrow_forward6. Match each of the lettered items in the column on the left with the most appropriate numbered item(s) in the column on the right. Some of the numbered items may be used more than once and some not at all. a. Z = 37 1. b. Mn 2. C. Pr element in period 5 and group 14 element in period 5 and group 15 d. S e. [Rn] 7s¹ f. d block metal 3. highest metallic character of all the elements 4. paramagnetic with 5 unpaired electrons 5. 4f36s2 6. isoelectronic with Ca²+ cation 7. an alkaline metal 8. an f-block elementarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

