
Concept explainers
(a)
Interpretation:
A Lewis structure for
Concept introduction:
Lewis structure is generally considered as a simplified structure of any molecule or atom. Lewis structure for any atom or molecule depicts the valence electrons as dots around the element’s symbol present in the molecule along with the bonds that connect them. Every element tries to complete an octet except the hydrogen atom.
Every element in the Lewis structure tries to attain eight electrons in its valence shell by transfer or share of electrons. This rule is known as the octet rule.
To draw the Lewis structure of the molecule there are following steps:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Step 5: Convert the lone pair into bond pair.
(a)
Answer to Problem 10.76P
The Lewis structure for
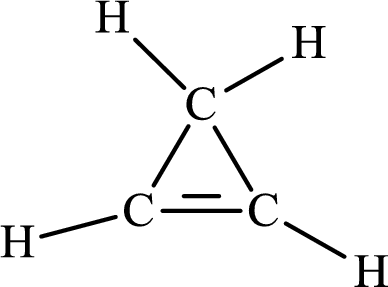
The carbon-carbon single bond has the bond order 1 and carbon-carbon double bond has the bond order 2.
Explanation of Solution
The total number of valence electrons of
Substitute 3 for the total number of
In
The Lewis structure of
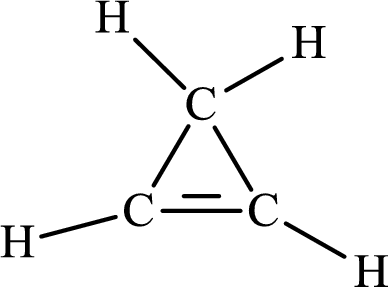
The single bond has the bond order 1 and double bond has the bond order 2. Therefore, the carbon-carbon single bond has the bond order 1 and carbon-carbon double bond has the bond order 2.
The single bond has the bond order 1, the double bond has the bond order 2 and triple bond has the bond order 3.
(b)
Interpretation:
A Lewis structure for
Concept introduction:
Lewis structure is generally considered as a simplified structure of any molecule or atom. Lewis structure for any atom or molecule depicts the valence electrons as dots around the element’s symbol present in the molecule along with the bonds that connect them. Every element tries to complete an octet except the hydrogen atom.
Every element in the Lewis structure tries to attain eight electrons in its valence shell by transfer or share of electrons. This rule is known as octet rule.
To draw the Lewis structure of the molecule there are following steps:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Step 5: Convert the lone pair into bond pair.
(b)
Answer to Problem 10.76P
The Lewis structure of
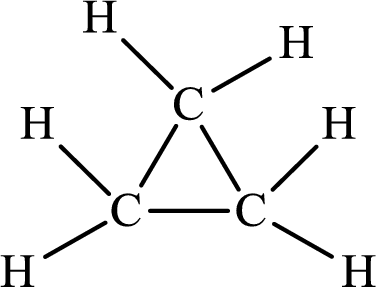
There is only single bond in
Explanation of Solution
The total number of valence electrons of
Substitute 3 for the total number of
In
The Lewis structure of
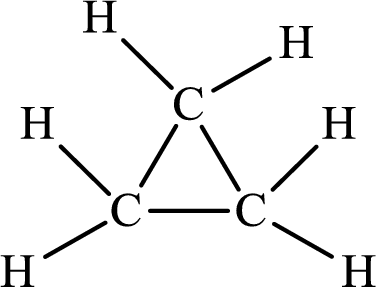
The single bond has the bond order 1 and double bond has the bond order 2. Therefore, there is only single bond in
The single bond has the bond order 1, double bond has the bond order 2 and triple bond has the bond order 3.
(c)
Interpretation:
A Lewis structure for
Concept introduction:
Lewis structure is generally considered as a simplified structure of any molecule or atom. Lewis structure for any atom or molecule depicts the valence electrons as dots around the element’s symbol present in the molecule along with the bonds that connect them. Every element tries to complete an octet except the hydrogen atom.
Every element in the Lewis structure tries to attain eight electrons in its valence shell by transfer or share of electrons. This rule is known as the octet rule.
To draw the Lewis structure of the molecule there are following steps:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Step 5: Convert the lone pair into bond pair.
(c)
Answer to Problem 10.76P
The Lewis structure of
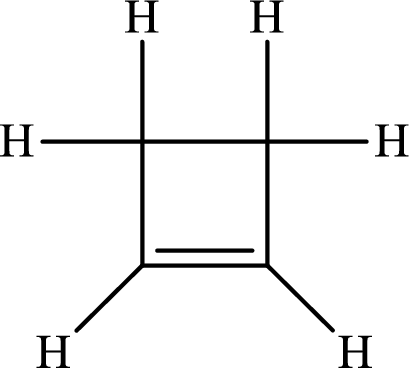
The carbon-carbon single bond has the bond order 1 and carbon-carbon double bond has the bond order 2.
Explanation of Solution
The total number of valence electrons of
Substitute 4 for the total number of
In
The Lewis structure of
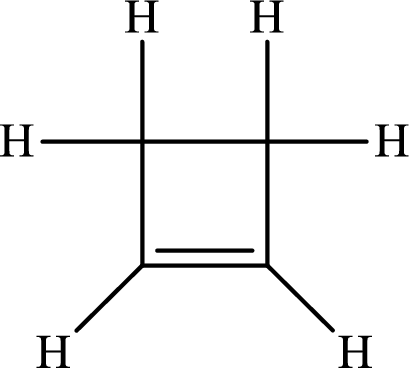
The single bond has the bond order 1 and double bond has the bond order 2. Therefore, the carbon-carbon single bond has the bond order 1 and carbon-carbon double bond has the bond order 2.
The single bond has the bond order 1, the double bond has the bond order 2 and triple bond has the bond order 3.
(d)
Interpretation:
A Lewis structure for
Concept introduction:
Lewis structure is generally considered as a simplified structure of any molecule or atom. Lewis structure for any atom or molecule depicts the valence electrons as dots around the element’s symbol present in the molecule along with the bonds that connect them. Every element tries to complete an octet except the hydrogen atom.
Every element in the Lewis structure tries to attain eight electrons in its valence shell by transfer or share of electrons. This rule is known as the octet rule.
To draw the Lewis structure of the molecule there are following steps:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Step 5: Convert the lone pair into bond pair.
(d)
Answer to Problem 10.76P
The Lewis structure of
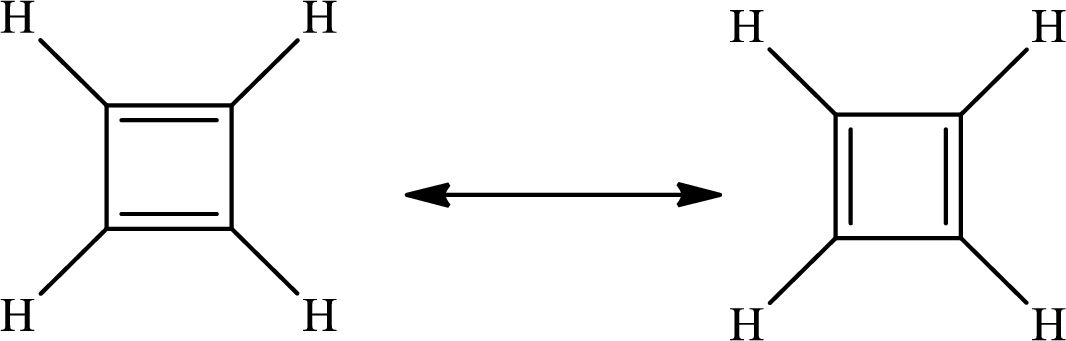
The average bond order in
Explanation of Solution
The total number of valence electrons of
Substitute 4 for the total number of
In
There is alternate single and double present in the ring so the molecule will show resonance.
The Lewis structure of
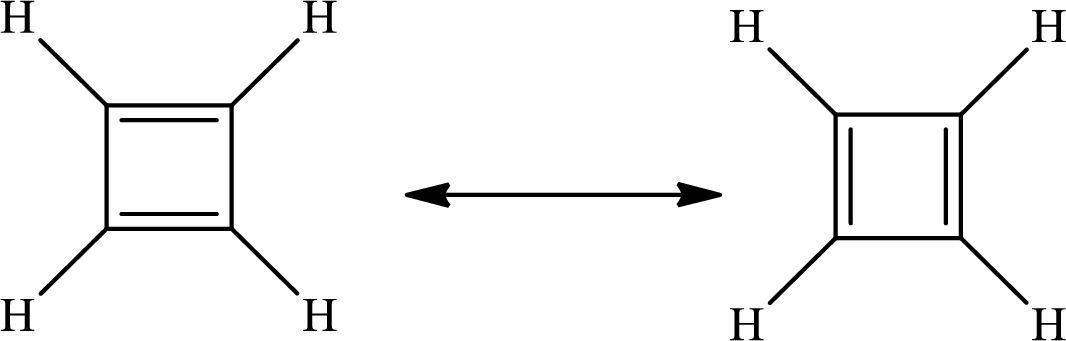
There are 4 single bonds and 2 double bonds in
The formula to calculate the average bond order in
Substitute 6 for the number of shared electron pair and 4 for the number of bonded atoms in equation (5).
The single bond has the bond order 1, the double bond has the bond order 2 and triple bond has the bond order 3.
(e)
Interpretation:
A Lewis structure for
Concept introduction:
Lewis structure is generally considered as a simplified structure of any molecule or atom. Lewis structure for any atom or molecule depicts the valence electrons as dots around the element’s symbol present in the molecule along with the bonds that connect them. Every element tries to complete an octet except the hydrogen atom.
Every element in the Lewis structure tries to attain eight electrons in its valence shell by transfer or share of electrons. This rule is known as the octet rule.
To draw the Lewis structure of the molecule there are following steps:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Step 5: Convert the lone pair into bond pair.
(e)
Answer to Problem 10.76P
The Lewis structure of
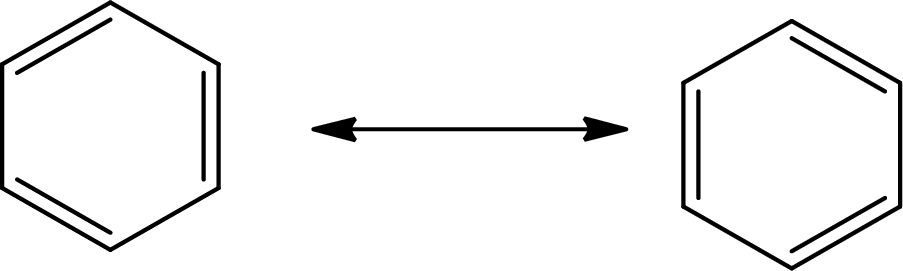
The average bond order in
Explanation of Solution
The total number of valence electrons of
Substitute 6 for the total number of
In
There is alternate single and double present in the ring so the molecule will show resonance.
The Lewis structure of
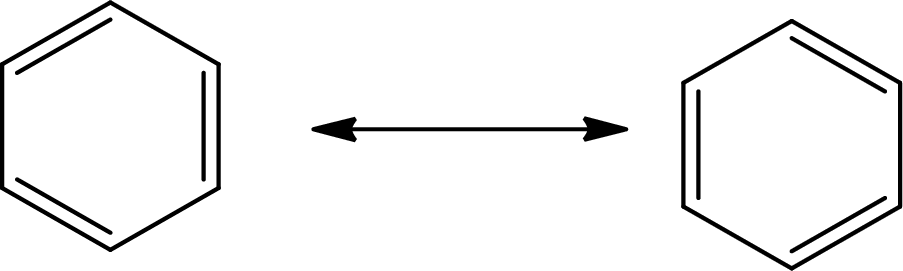
There are 6 single bonds and 3 double bonds in
The formula to calculate the average bond order in
Substitute 9 for the number of shared electron pair and 6 for the number of bonded atoms in equation (7).
The single bond has the bond order 1, the double bond has the bond order 2 and triple bond has the bond order 3.
Want to see more full solutions like this?
Chapter 10 Solutions
CHEMISTRY >CUSTOM<
- Liquid chromatography has been used to track the concentration of remdesivir (a broad-spectrum antiviral drug, structure shown at right) in COVID patients undergoing experimental treatments. Intensity The authors provide the following details regarding standard solutions preparation: HN CN HO OH NH2 Remdesivir (RDV) stock solution (5000 µg/mL) was prepared by dissolving RDV drug powder using the mixture of DMSO: MeOH (30:70 v/v). The RDV working standard solutions for calibration and quality controls were prepared using methanol in concentrations of 100, 10, 1, 0.1, 0.01 µg/mL. 1, 2.5, 5, 7.5, 10, 25, 50, 75, 100, 250, 500, 1000, and 5000 ng/mL sample solutions were prepared freshly by spiking calibration standard solutions into the blank human plasma samples for method calibration. a) What type of calibration method is being described? Why do you think the authors chose this method as opposed to another? b) Based on the details provided in part a, describe an appropriate method blank…arrow_forwardRecent advancements in liquid chromatography include the development of ultrahigh pressure liquid chromatography (UHPLC) and an increased use of capillary columns that had previously only been used with gas chromatography. Both of these advances have made the development of portable LC systems possible. For example, Axcend Corp. makes a portable system that uses a capillary column with an internal diameter of 150-μm-that is packed with 1.7-um stationary phase particles. In contrast, a traditional LC column has a 4.6 mm internal diameter and utilizes 5-um stationary phase particles. a) Explain one advantage that is afforded by the use of a capillary column in liquid chromatographic separation. Explain one disadvantage of capillary columns. b) Explain how the use of smaller stationary phase particles can improve the resolution of a separation. Include any relevant equations that support your explanation. c) A scientist at Rowan University is using the Axcend LC to conduct analyses of F…arrow_forwardThis paper describes the use of fullerene molecules, also known as buckyballs, as a stationary phase for liquid chromatography. The performance of the fullerene-modified stationary phase (FMS) is compared to that of a more common C18 stationary phase and to two other carbon-based stationary phases, PGC and COZ. A. 10A OM B. - Figure 1. Idealized drawing of the cross-section of a pore inside a silica particle, showing the relative densities of aminopropylsilyl (red/green) and fullerene (blue) groups: (A) full cross- section; (B) detailed view of covalent bonding of fullerene to the silica surface. Surface densities of silyl and fullerene groups were inferred from elemental composition results obtained at each stage of the synthesis (see Table 1). Absorbance (mAU, 220 nm) 700 600 500 400 300 200 100 a. Define selectivity, a, with words and an equation. b. Explain how the choice of stationary phase affects selectivity. c. Calculate the resolution of the nitrobenzene and toluene peaks in…arrow_forward
- Normalized Intensity (a. u.) 0.5 1.0 A 3D-printed GC column (shown below) was created for use with "micro" gas chromatography applications. To prove its utility, it was used to separate a mixture of alkanes (C9-C18, C22, C24). For the separation shown below, the column temperature was ramped from 40 °C to 250 °C at a rate of 30 °C per minute. (a) 9 10 = 1 mm 12 13 15 22 0.0 0 100 200 300 400 Time (sec) a) What detector would you use for this analysis? Justify your selection. b) Explain how the chromatogram would change if the separation was run isothermally. c) Explain how the chromatogram would change if the temperature ramp were increased to 50 °C per minute.arrow_forwardDevise a synthesis of each compound from the indicated starting material. You may also use any organic compounds with one or two carbons and any needed inorganic reagents. a. Brarrow_forwardPlease help me with #2b & #3 using the data.arrow_forward
- Heparin is used as an anti-coagulant. A risk of heparin use is thrombocytopenia, or low platelet count. This risk is minimized with the use of low molecular weight heparins (LMWH), therefore it is desirable to separate LMWH from higher molecular weight heparins. The method of choice to do this is molecular exclusion chromatography. Below is a chromatogram from a molecular exclusion chromatographic run. Peaks ranging from A to J are clearly distinguishable. The heparin mixture that was analyzed had anywhere from 6 to 30 repeat units of monomer (where the heparin with 30 repeat units would be roughly five times the size of the heparin with six repeat units). a. Which letter most likely represents the peak with 6 repeat units given these heparin polymers were separated with molecular exclusion chromatography? b. Explain your reasoning describing the mechanism of retention in molecular exclusion chromatography. 100 80 60 60 Relative Abundance 40 40 E GH 20 20 B A 36 38 40 42 44 46 48 50 50…arrow_forwardHELP NOW PLEASE ! URGENT!arrow_forwardHELP NOW PLEASE ! URGENT!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





