
Concept explainers
(a)
Interpretation:
The molecular shape of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(a)
Answer to Problem 10.63P
The molecular shape of
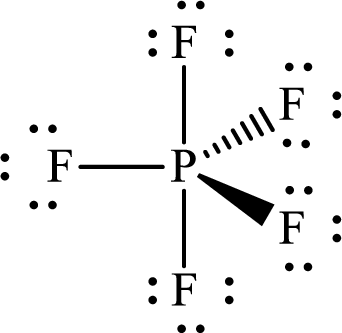
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
Thus, the Lewis structure of
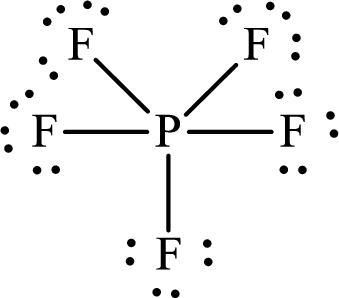
According to the Lewis structure of
The electron-group arrangement around the central atom in
(b)
Interpretation:
The molecular shape of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(b)
Answer to Problem 10.63P
The molecular shape of
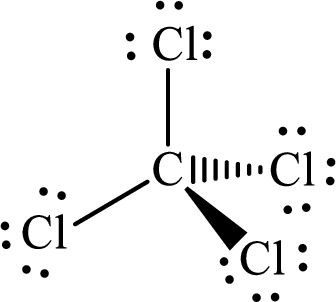
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
The Lewis structure of
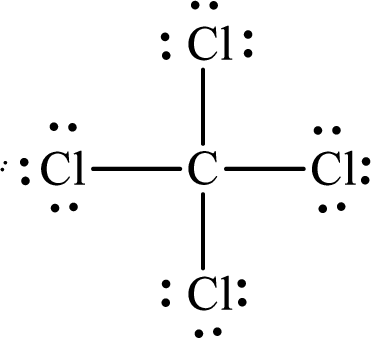
According to the Lewis structure of
The electron-group arrangement around the central atom in
(c)
Interpretation:
The molecular shape of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(c)
Answer to Problem 10.63P
The molecular shape of
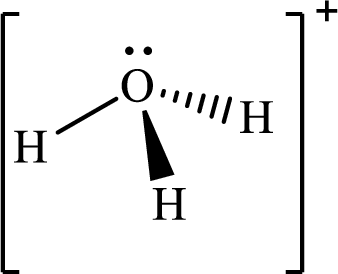
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
The total number of valence electrons in
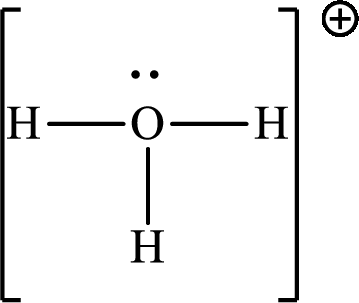
According to the Lewis structure of
The electron-group arrangement around the central atom in
(d)
Interpretation:
The molecular shape of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(d)
Answer to Problem 10.63P
The molecular shape of
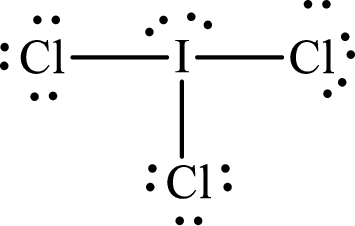
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
With
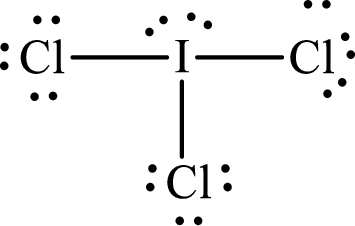
According to the Lewis structure of
The electron-group arrangement around the central atom in
(e)
Interpretation:
The molecular shape of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(e)
Answer to Problem 10.63P
The molecular shape of
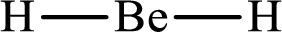
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
With
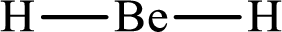
According to the Lewis structure of
The electron-group arrangement around the central atom in
(f)
Interpretation:
The molecular shape of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(f)
Answer to Problem 10.63P
The molecular shape of
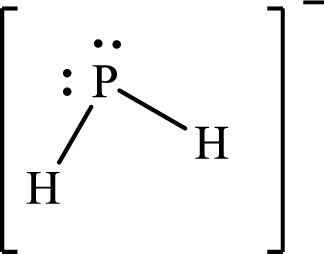
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
The Lewis structure of
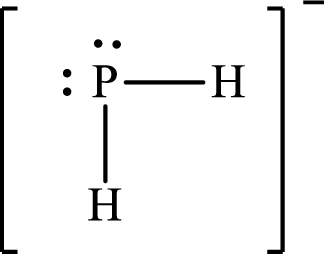
According to the Lewis structure of molecular shape of
The electron-group arrangement around the central atom in
(g)
Interpretation:
The molecular shape of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(g)
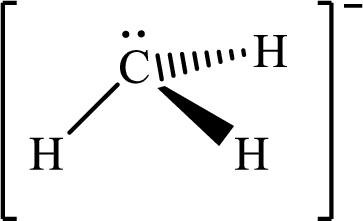
Answer to Problem 10.63P
The molecular shape of
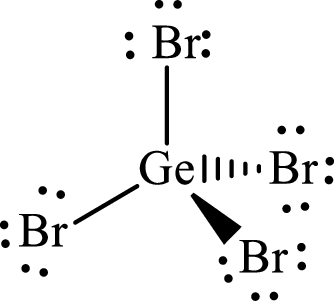
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
The total number of valence electrons in
According to the Lewis structure of
The electron-group arrangement around the central atom in
(h)
Interpretation:
The molecular shape of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(h)
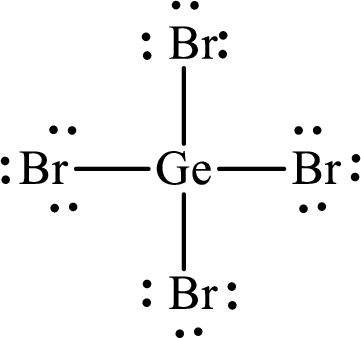
Answer to Problem 10.63P
The molecular shape of
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
The total number of valence electrons in
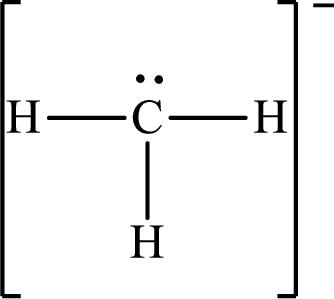
According to the Lewis structure of
The electron-group arrangement around the central atom in
(i)
Interpretation:
The molecular shape of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(i)
Answer to Problem 10.63P
The molecular shape of
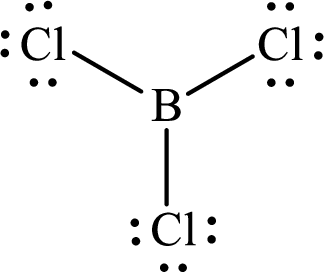
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
These 24 electrons are placed such that three of these form bonding pairs and the remaining ones reside as lone pairs as shown below:
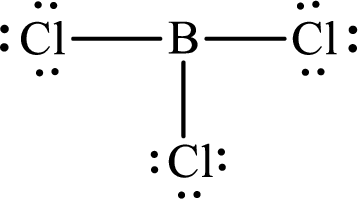
According to the Lewis structure of
The electron-group arrangement around the central atom in
(j)
Interpretation:
The molecular shape of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(j)
Answer to Problem 10.63P
The molecular shape of
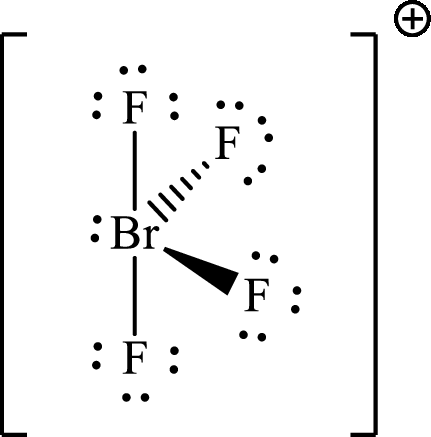
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
The Lewis structure for
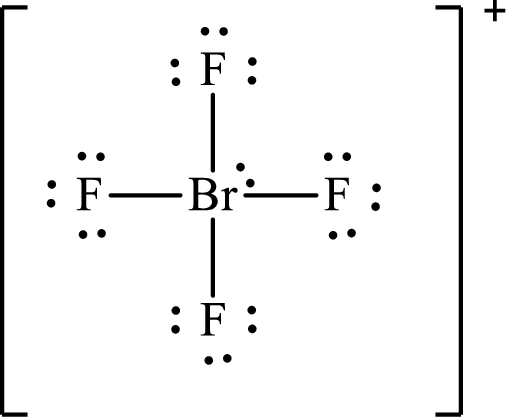
According to the Lewis structure of
The electron-group arrangement around the central atom in
(k)
Interpretation:
The molecular shape of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(k)
Answer to Problem 10.63P
The molecular shape of
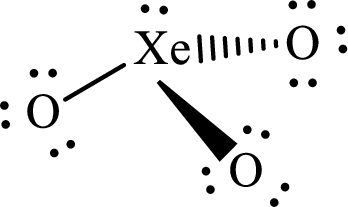
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
The total number of valence electrons in
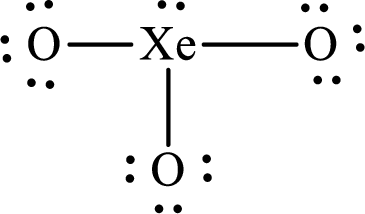
According to the Lewis structure of
The electron-group arrangement around the central atom in
(l)
Interpretation:
The molecular shape of
Concept introduction:
The steps to draw the Lewis structure of the given molecule are as follows:
Step 1: Choose the least electronegative central metal atom and place the atoms relative to each other.
Step 2: Determine the total number of valence electron.
Step 3: Place a single electron pair between each atom and subtract 2 electrons corresponding to each of these bonds from the total number of valence electrons.
Step 4: Distribute the remaining electrons in pairs around each atom as non bonding electrons such that each atom gets a complete share of eight electrons.
(l)
Answer to Problem 10.63P
The molecular shape of
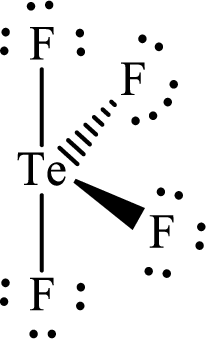
Explanation of Solution
The total number of valence electrons of
Substitute 1 for the total number of
Analogous to
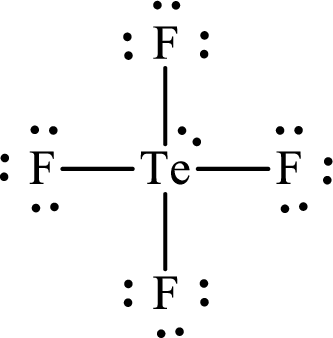
According to the Lewis structure of
The electron-group arrangement around the central atom in
Want to see more full solutions like this?
Chapter 10 Solutions
CHEMISTRY >CUSTOM<
- Draw the major product of this reaction.arrow_forwardidentify the carbonyl compound that is incapable of forming an enolate ionarrow_forwardpredict the product formed by the reaction of one mole each of cyclohex-2-en-1-one and lithium diethylcuprate. Assume a hydrolysis step follows the additionarrow_forward
- Please handwriting for questions 1 and 3arrow_forwardIs (CH3)3NHBr an acidic or basic salt? What happens when dissolved in aqueous solution? Doesn't it lose a Br-? Does it interact with the water? Please advise.arrow_forward© Macmilla Finish resonance structure 3 Select Draw Templates More C H N 0 H H S Erase Which structure is the most stable (lowest energy) resonance contributor? The structure with the positive charge on nitrogen and negative charges on oxygen and sulfur. All structures are equal in stability. The structure with the positive charge on nitrogen and negative charges on sulfur and carbon. The structure with the positive charge on nitrogen and negative charges on oxygen and carbon. Q2Qarrow_forward
- Three pure compounds are formed when 1.00 g samples of element x combine with, respectively, 0.472 g, 0.630 g, and 0.789 g of element z. The first compound has the formula x2Z3. find the empricial formula of the other two compoundsarrow_forwardDraw the product and the mechanism A. excess H*; 人 OH H*; B. C. D. excess OH ✓ OH H*; H₂O 1. LDA 2. H*arrow_forwardIn reactions whose kinetic equation is v = k[A]m, the rate coefficient k is always positive. Is this correct?arrow_forward
- If the concentration of A decreases exponentially with time, what is the rate equation? (A). -d[A] (B). dt d[A] = k[A] e-kt dtarrow_forwardGiven the first-order reaction: aA → products. State its kinetic equation.arrow_forwardDetermine the symmetry of the combination of atomic orbitals for bf 4-arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





